Prilocaine hydrochloride: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{KS}} | |authorTag={{KS}} | ||
|genericName=prilocaine hydrochloride | |||
|aOrAn=a | |aOrAn=a | ||
|adverseReactions= | |drugClass=local anesthetic | ||
|indicationType=procedure | |||
|indication=production of local anesthesia | |||
|adverseReactions= [[bradycardia]], [[hypotension]], [[urticaria]], [[edema]], [anaphylactoid reaction]]s, | |||
[[lightheadedness]], [[nervousness]], [[euphoria]], [[confusion]], [[dizziness]], [[drowsiness]], [[tinnitus]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 34: | Line 39: | ||
* In patients weighing <150 lbs (70 kg), no more than 4 mg/lb (8 mg/kg) should be administered. In patients weighing <150 lbs, no more than 600 mg (8 cartridges) of prilocaine HCl should be administered as a single injection. | * In patients weighing <150 lbs (70 kg), no more than 4 mg/lb (8 mg/kg) should be administered. In patients weighing <150 lbs, no more than 600 mg (8 cartridges) of prilocaine HCl should be administered as a single injection. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 97: | Line 101: | ||
* The incidences of adverse reactions (e.g., persistent neurologic deficit) associated with the use of local anesthetics may be related to the technique employed, the total dose of local anesthetic administered, the particular drug used, the route of administration, and the physical condition of the patient. | * The incidences of adverse reactions (e.g., persistent neurologic deficit) associated with the use of local anesthetics may be related to the technique employed, the total dose of local anesthetic administered, the particular drug used, the route of administration, and the physical condition of the patient. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|drugInteractions=* Concurrent administration of vasopressor drugs and ergot-type oxytocic drugs may cause severe, persistent [[hypertension]] or cerebrovascular accidents. | |drugInteractions=* Concurrent administration of vasopressor drugs and ergot-type oxytocic drugs may cause severe, persistent [[hypertension]] or cerebrovascular accidents. | ||
* Prilocaine may contribute to the formation of methemoglobinemia in patients treated with other drugs known to cause this condition | * Prilocaine may contribute to the formation of methemoglobinemia in patients treated with other drugs known to cause this condition | ||
|useInPregnancyFDA='''Pregnancy Category B''' | |useInPregnancyFDA='''Pregnancy Category B''' | ||
| Line 111: | Line 113: | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when prilocaine is administered to a nursing woman. | |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when prilocaine is administered to a nursing woman. | ||
|useInPed=* Dosages in children should be reduced, commensurate with age, body weight, and physical condition. | |useInPed=* Dosages in children should be reduced, commensurate with age, body weight, and physical condition. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
Revision as of 19:43, 2 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Prilocaine hydrochloride is a local anesthetic that is FDA approved for the procedure of production of local anesthesia. Common adverse reactions include bradycardia, hypotension, urticaria, edema, [anaphylactoid reaction]]s, lightheadedness, nervousness, euphoria, confusion, dizziness, drowsiness, tinnitus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- 4% Citanest Plain Dental Injection is indicated for the production of local anesthesia in dentistry by nerve block or infiltration techniques.
Dosage
- The dosage of 4% Citanest Plain Dental Injection varies and depends on the physical status of the patient, the area of the oral cavity to be anesthetized, the vascularity of the oral tissues, and the technique of anesthesia. The least volume of injection that results in effective local anesthesia should be administered. For specific techniques and procedures of local anesthesia in the oral cavity, refer to standard textbooks.
Inferior Alveolar Block
- There are no practical clinical differences between prilocaine with and without epinephrine when used for inferior alveolar blocks.
Maxillary Infiltration
- 4% Citanest Plain Dental Injection is recommended for use in maxillary infiltration anesthesia for procedures in which the painful aspects can be completed within 15 minutes after the injection. 4% Citanest Plain Dental Injection is therefore especially suited to short procedures in the maxillary anterior teeth. For long procedures, or those involving maxillary posterior teeth where soft tissue numbness is not troublesome to the patient, Prilocaine HCl 4% with epinephrine 1:200,000 is recommended.
- For most routine procedures, initial dosages of 1 to 2 mL of 4% Citanest Plain Dental Injection will usually provide adequate infiltration or major nerve block anesthesia.
- The maximum recommended dose that should ever be administered within a two-hour period in normal healthy adults should be calculated based upon the patient's weight as follows:
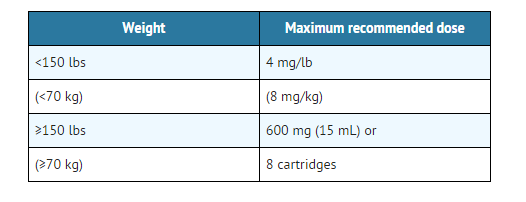
Maximum Recommended Dosages
- In patients weighing <150 lbs (70 kg), no more than 4 mg/lb (8 mg/kg) should be administered. In patients weighing <150 lbs, no more than 600 mg (8 cartridges) of prilocaine HCl should be administered as a single injection.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prilocaine hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Prilocaine hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Prilocaine hydrochloride in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prilocaine hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Prilocaine hydrochloride in pediatric patients.
Contraindications
- Prilocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type and in those rare patients with congenital or idiopathic methemoglobinemia.
Warnings
- DENTAL PRACTITIONERS WHO EMPLOY LOCAL ANESTHETIC AGENTS SHOULD BE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF EMERGENCIES THAT MAY ARISE FROM THEIR USE. RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS SHOULD BE AVAILABLE FOR IMMEDIATE USE.
- To minimize the likelihood of intravascular injection, aspiration should be performed before the local anesthetic solution is injected. If blood is aspirated, the needle must be repositioned until no return of blood can be elicited by aspiration. Note, however, that the absence of blood in the syringe does not assure that intravascular injection will be avoided.
Methemoglobinemia
- Prilocaine has been associated with the development of methemoglobinemia. Very young patients, patients with congenital or idiopathic methemoglobinemia, or patients with glucose-6-phosphate deficiencies are more susceptible to methemoglobinemia.
- Patients taking drugs associated with drug induced methemoglobinemia such as sulfonamides, acetaminophen, acetanilid, aniline dyes, benzocaine, chloroquine, dapsone, napthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, para-aminosalicylic acid, phenacetin, phenobarbital, phenytoin, primaquine, and quinine are also at greater risk for developing methemoglobinemia.
PRECAUTIONS
General
- The safety and effectiveness of prilocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for specific techniques and precautions for various regional anesthetic procedures. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use.The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Repeated doses of prilocaine may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug or its metabolites. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical status. Prilocaine should also be used with caution in patients with severe shock or heart block.
- Cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be monitored after each local anesthetic injection. Restlessness, anxiety, tinnitus, dizziness, blurred vision, tremors, depression or drowsiness should alert the practitioner to the possibility of central nervous system toxicity. Signs and symptoms of depressed cardiovascular function may commonly result from a vasovagal reaction, particularly if the patient is in an upright position.
- Since amide-type local anesthetics are metabolized by the liver, prilocaine should be used with caution in patients with hepatic disease.
- Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations. Prilocaine should also be used with caution in patients with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
- Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Since it is not known whether amide-type local anesthetics may trigger this reaction and since the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for the management of malignant hyperthermia should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure, and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene (consult dantrolene sodium intravenous package insert before using).
- Prilocaine should be used with caution in persons with known drug sensitivities. Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to prilocaine.
Use in the Head and Neck Area
- Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded.
Adverse Reactions
Clinical Trials Experience
- Swelling and persistent paresthesia of the lips and oral tissues may occur. Persistent paresthesias lasting weeks to months, and in rare instances paresthesia lasting greater than one year, have been reported.
- Adverse experiences following the administration of prilocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage, rapid absorption or unintentional intravascular injection, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central Nervous System
- CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression, and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest.
- Drowsiness following the administration of prilocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular System
- Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
- Signs and symptoms of depressed cardiovascular function may commonly result from a vasovagal reaction, particularly if the patient is in an upright position. Less commonly, they may result from a direct effect of the drug. Failure to recognize the premonitory signs such as sweating, a feeling of faintness, changes in pulse or sensorium may result in progressive cerebral hypoxia and seizure or serious cardiovascular catastrophe. Management consists of placing the patient in the recumbent position and ventilation with oxygen. Supportive treatment of circulatory depression may require the administration of intravenous fluids, and, when appropriate, a vasopressor (e.g., ephedrine) as directed by the clinical situation.
Allergic
Allergic reactions are characterized by cutaneous lesions, urticaria, edema, or anaphylactoid reactions. Allergic reactions as a result of sensitivity to prilocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
Neurologic
- The incidences of adverse reactions (e.g., persistent neurologic deficit) associated with the use of local anesthetics may be related to the technique employed, the total dose of local anesthetic administered, the particular drug used, the route of administration, and the physical condition of the patient.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Prilocaine hydrochloride in the drug label.
Drug Interactions
- Concurrent administration of vasopressor drugs and ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
- Prilocaine may contribute to the formation of methemoglobinemia in patients treated with other drugs known to cause this condition
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category B
- Reproduction studies have been performed in rats at doses up to 30 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to prilocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering prilocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Prilocaine hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Prilocaine hydrochloride during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when prilocaine is administered to a nursing woman.
Pediatric Use
- Dosages in children should be reduced, commensurate with age, body weight, and physical condition.
Geriatic Use
There is no FDA guidance on the use of Prilocaine hydrochloride with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Prilocaine hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Prilocaine hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Prilocaine hydrochloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Prilocaine hydrochloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Prilocaine hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Prilocaine hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Prilocaine hydrochloride in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Prilocaine hydrochloride in the drug label.
Overdosage
- Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics.
Management of Local Anesthetic Emergencies
- The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
- The first step in the management of convulsions consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as directed by the clinical situation (eg, ephedrine).
- If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
- Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or controlled) is indicated.
- Dialysis is of negligible value in the treatment of acute overdosage with prilocaine.
- The development of methemoglobinemia is generally dose related but may occur at any dose in susceptible individuals. While methemoglobin values of less than 20% do not generally produce any clinical symptoms, the appearance of cyanosis at 2 to 4 hours following administration should be evaluated in terms of the general health status of the patient.
- Methemoglobinemia can be reversed when indicated by intravenous administration of methylene blue at a dosage of 1 to 2 mg/kg given over a five minute period.
- The subcutaneous LD50 of prilocaine HCl in female mice is 550 (359 to 905) mg/kg.
Pharmacology
There is limited information regarding Prilocaine hydrochloride Pharmacology in the drug label.
Mechanism of Action
- Prilocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Onset and Duration of Action
- When used for infiltration injection in dental patients, the time of onset of anesthesia averages less than 2 minutes with an average duration of soft tissue anesthesia of approximately 2 hours.
- Based on electrical stimulation studies, 4% Citanest Plain Dental Injection provides a duration of pulpal anesthesia of approximately 10 minutes in maxillary infiltration injections. In clinical studies, this has been found to provide complete anesthesia for procedures lasting an average of 20 minutes.
- When used for inferior alveolar nerve block, the time of onset of 4% Citanest Plain Dental Injection averages less than three minutes with an average duration of soft tissue anesthesia of approximately 2½ hours.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Prilocaine hydrochloride in the drug label.
Pharmacokinetics
- Information derived from diverse formulations, concentrations and usages reveals that prilocaine is completely absorbed following parenteral administration, its rate of absorption depending, for example, upon such factors as the site of administration and the presence or absence of a vasoconstrictor agent. Prilocaine is metabolized in both the liver and the kidney and excreted via the kidney. It is not metabolized by plasma esterases. Hydrolysis of prilocaine by amidases yields ortho-toluidine and N-proylalanine. Both of these compounds may undergo ring hydroxylation.
- O-toluidine has been found to produce methemoglobin, both in vitro and in vivo.
- Because prilocaine is metabolized in both the liver and kidneys, hepatic and renal dysfunction may alter prilocaine kinetics.
- As with other local anesthetic agents, the plasma binding of prilocaine may be dependent on drug concentration. At 0.5 to 1.0 mg/mL it is 55% protein bound.
- Prilocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
- Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of prilocaine required to produce overt systemic effects. In the rhesus monkey, arterial blood levels of 20 mg/mL have been shown to be the threshold for convulsive activity.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Studies of prilocaine in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
- Chronic oral toxicity studies of ortho-toluidine, a metabolite of prilocaine, in mice (150 to 4800 mg/kg) and rats (150 to 800 mg/kg) have shown that ortho-toluidine is a carcinogen in both species. The lowest dose corresponds to approximately 50 times the maximum amount of ortho-toluidine to which a 50 kg subject would be expected to be exposed following a single injection (8 mg/kg) of prilocaine.
- Ortho-toluidine (0.5 mg/mL) showed positive results in Escherichia coli DNA repair and phage-induction assays. Urine concentrates from rats treated with ortho-toluidine (300 mg/kg, orally) were mutagenic for Salmonella typhimurium with metabolic activation. Several other tests, including reverse mutations in five different Salmonella typhimurium strains with or without metabolic activation and single strand breaks in DNA of V79 Chinese hamster cells, were negative.
Clinical Studies
There is limited information regarding Clinical Studies of Prilocaine hydrochloride in the drug label.
How Supplied
- 4% Citanest Plain Dental Injection (NDC 66312-630-14) is dispensed in 1.8 mL cartridges, packed 50 per box.
Storage
There is limited information regarding Prilocaine hydrochloride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Prilocaine hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Prilocaine hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- The patient should be informed of the possibility of temporary loss of sensation and muscle function following infiltration or nerve block injections.
- The patient should be advised to exert caution to avoid inadvertent trauma to the lips, tongue, cheek mucosae, or soft palate when these structures are anesthetized. The ingestion of food should therefore be postponed until normal function returns.
- The patient should be advised to consult the dentist if anesthesia persists, or if a rash develops.
Precautions with Alcohol
- Alcohol-Prilocaine hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Prilocaine hydrochloride
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Prilocaine hydrochloride |Label Name=Prilocaine hydrochloride11.png
}}
{{#subobject:
|Label Page=Prilocaine hydrochloride |Label Name=Prilocaine hydrochloride11.png
}}