Pravastatin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{SS}} | ||
|genericName=Pravastatin | |||
|aOrAn=a | |||
|drugClass=HMG-COA Reductase Inhibitor | |||
|indicationType=treatment | |||
|indication=[[hypercholesterolemia]], [[prevention of cardiovascular disease]] | |||
|adverseReactions=[[Rash]], [[Diarrhea]], [[Nausea]] and [[vomiting]], Musculoskeletal pain, [[Headache]], [[Cough]], [[Rhinitis]], [[Upper respiratory infection]] | |||
|fdaLIADAdult======[[Hypercholesterolemia]]===== | |||
* Indication | |||
:* Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to [[hypercholesterolemia]]. Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. | |||
====[[Prevention of Cardiovascular Disease]]==== | |||
* Indication | |||
:* In hypercholesterolemic patients without clinically evident [[coronary heart disease]] (CHD), pravastatin sodium tablets USP are indicated to: | |||
::* reduce the risk of [[myocardial infarction]] ([[MI]]). | |||
::* reduce the risk of undergoing myocardial revascularization procedures. | |||
::* reduce the risk of cardiovascular mortality with no increase in death from non-cardiovascular causes. | |||
* General Dosing Information | |||
:* The patient should be placed on a standard cholesterol-lowering diet before receiving pravastatin sodium tablets and should continue on this diet during treatment with pravastatin sodium tablets [see NCEP Treatment Guidelines for details on dietary therapy]. | |||
* Recommended starting dosage: ''' 40 mg PO qd'''. | |||
:* If a daily dose of '''40 mg''' does not achieve desired cholesterol levels, '''80 mg once daily''' is recommended. | |||
:* In patients with significant renal impairment, | |||
::* Starting dosage: '''10 mg PO qd'''. Pravastatin sodium tablets can be administered orally as a single dose at any time of the day, with or without food. | |||
::* Since the maximal effect of a given dose is seen within 4 weeks, periodic lipid determinations should be performed at this time and dosage adjusted according to the patient’s response to therapy and established treatment guidelines. | |||
<H4>Concomitant Lipid-Altering Therapy</H4> | |||
* Dosing information | |||
:* Pravastatin sodium tablets may be used with bile acid resins. When administering a bile-acid-binding resin (e.g., cholestyramine, colestipol) and pravastatin, pravastatin sodium tablets should be given either 1 hour or more before or at least 4 hours following the resin. [See Clinical Pharmacology (12.3).] | |||
<H4>Dosage in Patients Taking [[Cyclosporine]]</H4> | |||
* Dosing information | |||
:* In patients taking immunosuppressive drugs such as [[cyclosporine]] concomitantly with pravastatin, therapy should begin with 10 mg of pravastatin sodium once-a-day at bedtime and titration to higher doses should be done with caution. | |||
:* Most patients treated with this combination received a maximum pravastatin sodium dose of '''20 mg/day'''. | |||
:* In patients taking [[cyclosporine]]: less than '''20 mg PO qd''' [see Warnings and Precautions (5.1) and Drug Interactions (7.1)]. | |||
<H4>Dosage in Patients Taking [[Clarithromycin]]</H4> | |||
* Dosing information | |||
:* In patients taking [[clarithromycin]]: less than '''40 mg PO qd''' [see Drug Interactions (7.2)]. | |||
| | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=<H4>[[Acute coronary syndrome]]</H4> | |||
* Dosing information | |||
:* | |||
< | <h4>[[Atrial fibrillation]]</h4> | ||
* Dosing information | |||
:* '''80 mg/day'''18242276 | |||
<h4>[[Coronary artery bypass graft]]</h4> | |||
* Dosing information | |||
:* '''10-20 mg.day''' 11074212 | |||
<H4>[[Heart failure]]</H4> | |||
* Dosing information | |||
:* '''HMG-CoA-reductase inhibitor (statin) therapy reduced the risks of all-cause mortality and hospitalization for heart failure among patients with chronic heart failure'''11074212 | |||
<H4>Kidney disease</H4> | |||
* Dosing information | |||
:* '''10 mg/day'''12105140 | |||
<H4>[[Nephrotic syndrome]]</H4> | |||
* Dosing information | |||
:* '''20 mg PO bid'''10644862 | |||
<H4>[[Percutaneous coronary intervention]]</H4> | |||
* Dosing information | |||
:* ''' 40 mg/day'''8840836 | |||
<H4>[[Radiographic contrast agent nephropathy]]</H4> | |||
* Dosing information | |||
:* ''' 10 mg/day''' 19782255 | |||
<H4>Restenotic lesion of coronary artery</H4> | |||
* Dosing information | |||
:* ''' 40 mg PO qd''' 11018193 | |||
<h4>[[Transient ischemic attack]]</h4> | |||
* Dosing information | |||
:* ''' 20 mg/day''' 11222801 | |||
|fdaLIADPed=<H4>[[Hypercholesterolemia]]</H4> | |||
* Dosing information | |||
:* Children (Ages 8 to 13 Years, Inclusive) | |||
::* Recommended dose: '''20 mg PO qd''' in children 8 to 13 years of age. Doses greater than 20 mg have not been studied in this patient population. | |||
:* Adolescents (Ages 14 to 18 Years) | |||
::* Recommended starting dose: '''40 mg PO qd''' in adolescents 14 to 18 years of age. Doses greater than 40 mg have not been studied in this patient population. | |||
::* Children and adolescents treated with pravastatin should be reevaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult goals for LDL-C [see Indications and Usage (1.2)]. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
==== | |contraindications====[[Hypersensitivity]]=== | ||
[[Hypersensitivity]] to any component of this medication. | |||
===Liver=== | |||
Active liver disease or unexplained, persistent elevations of serum transaminases [see Warnings and Precautions (5.2)]. | |||
===Pregnancy=== | |||
[[Atherosclerosis]] is a chronic process and discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary [[hypercholesterolemia]]. [[cholesterol]] and other products of [[cholesterol]] biosynthesis are essential components for fetal development (including synthesis of steroids and cell membranes). Since statins decrease [[cholesterol]] synthesis and possibly the synthesis of other biologically active substances derived from [[cholesterol]], they are contraindicated during pregnancy and in nursing mothers. '''PRAVASTATIN SHOULD BE ADMINISTERED TO WOMEN OF CHILDBEARING AGE ONLY WHEN SUCH PATIENTS ARE HIGHLY UNLIKELY TO CONCEIVE AND HAVE BEEN INFORMED OF THE POTENTIAL HAZARDS'''. If the patient becomes pregnant while taking this class of drug, therapy should be discontinued immediately and the patient apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)]. | |||
=== | ===Nursing Mothers=== | ||
A small amount of pravastatin is excreted in human breast milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require pravastatin sodium treatment should not breast-feed their infants [see Use in Specific Populations (8.3)]. | |||
|warnings====Skeletal Muscle=== | |||
Rare cases of [[rhabdomyolysis]] with [[acute renal failure]] secondary to [[myoglobinuria]] have been reported with pravastatin and other drugs in this class. A history of renal impairment may be a risk factor for the development of [[rhabdomyolysis]]. Such patients merit closer monitoring for skeletal muscle effects. | |||
Uncomplicated myalgia has also been reported in pravastatin-treated patients [see Adverse Reactions (6)]. [[Myopathy]], defined as muscle aching or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values to greater than 10 times the upper limit of normal (ULN), was rare (<0.1%) in pravastatin clinical trials. [[Myopathy]] should be considered in any patient with diffuse [[myalgias]], muscle tenderness or weakness, and/or marked elevation of CPK. Predisposing factors include advanced age (>65), uncontrolled [[hypothyroidism]], and renal impairment. | |||
There have been rare reports of [[immune-mediated necrotizing myopathy]] (IMNM), an autoimmune [[myopathy]], associated with statin use. [[IMNM]] is characterized by: proximal muscle weakness and elevated serum CPK, which persist despite discontinuation of statin treatment; muscle biopsy showing [[necrotizing myopathy]] without significant inflammation and improvement with immunosuppressive agents. | |||
All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by [[malaise]] or [[fever]] or if muscle signs and symptoms persist after discontinuing pravastatin sodium. | |||
Pravastatin therapy should be discontinued if markedly elevated CPK levels occur or [[myopathy]] is diagnosed or suspected. Pravastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of [[renal failure secondary]] to [[rhabdomyolysis]], e.g., [[sepsis]]; [[hypotension]]; major surgery; [[trauma]]; severe metabolic, [[endocrine]], or electrolyte disorders; or uncontrolled [[epilepsy]]. | |||
The risk of [[myopathy]] during treatment with [[statins]] is increased with concurrent therapy with either [[erythromycin]], [[cyclosporine]], [[niacin]], or [[fibrates]]. However, neither [[myopathy]] nor significant increases in CPK levels have been observed in 3 reports involving a total of 100 post-transplant patients (24 renal and 76 cardiac) treated for up to 2 years concurrently with pravastatin 10 to 40 mg and [[cyclosporine]]. Some of these patients also received other concomitant immunosuppressive therapies. Further, in clinical trials involving small numbers of patients who were treated concurrently with pravastatin and [[niacin]], there were no reports of [[myopathy]]. Also, [[myopathy]] was not reported in a trial of combination pravastatin (40 mg/day) and [[gemfibrozil]] (1200 mg/day), although 4 of 75 patients on the combination showed marked CPK elevations versus 1 of 73 patients receiving placebo. There was a trend toward more frequent CPK elevations and patient withdrawals due to musculoskeletal symptoms in the group receiving combined treatment as compared with the groups receiving placebo, [[gemfibrozil]], or pravastatin monotherapy. The use of [[fibrates]] alone may occasionally be associated with [[myopathy]]. The benefit of further alterations in lipid levels by the combined use of pravastatin sodiumwith fibrates should be carefully weighed against the potential risks of this combination. | |||
Cases of [[myopathy]], including [[rhabdomyolysis]], have been reported with pravastatin coadministered with [[colchicine]], and caution should be exercised when prescribing pravastatin with [[colchicine]] [see Drug Interactions (7.3)]. | |||
=== | ===Liver=== | ||
[[Statins]], like some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. In placebo-controlled clinical trials subjects were exposed to pravastatin or placebo [see Clinical Studies (14)]. In an analysis of serum transaminase values (ALT, AST), incidences of marked abnormalities were compared between the pravastatin and placebo treatment groups; a marked abnormality was defined as a post-treatment test value greater than 3 times the upper limit of normal for subjects with pretreatment values less than or equal to the upper limit of normal, or 4 times the pretreatment value for subjects with pretreatment values greater than the upper limit of normal but less than 1.5 times the upper limit of normal. Marked abnormalities of ALT or AST occurred with similar low frequency (≤1.2%) in both treatment groups. Overall, clinical trial experience showed that liver function test abnormalities observed during pravastatin therapy were usually asymptomatic, not associated with cholestasis, and did not appear to be related to treatment duration. In a 320-patient placebo-controlled clinical trial, subjects with chronic (>6 months) stable liver disease, due primarily to [[hepatitis C]] or nonalcoholic fatty liver disease, were treated with 80 mg pravastatin or placebo for up to 9 months. The primary safety endpoint was the proportion of subjects with at least one ALT ≥2 times the upper limit of normal for those with normal ALT (≤ the upper limit of normal) at baseline or a doubling of the baseline ALT for those with elevated ALT (> the upper limit of normal) at baseline. By Week 36, 12 out of 160 (7.5%) subjects treated with pravastatin met the prespecified safety ALT endpoint compared to 20 out of 160 (12.5%) subjects receiving placebo. Conclusions regarding liver safety are limited since the study was not large enough to establish similarity between groups (with 95% confidence) in the rates of ALT elevation. | |||
It is recommended that liver function tests be performed prior to the initiation of therapy and when clinically indicated. | |||
Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of pravastatin [see Contraindications (4.2)]. Caution should be exercised when pravastatin is administered to patients who have a recent (<6 months) history of liver disease, have signs that may suggest liver disease (e.g., unexplained aminotransferase elevations, jaundice), or are heavy users of alcohol. | |||
There have been rare postmarketing reports of fatal and non-fatal [[hepatic failure]] in patients taking [[statins]], including pravastatin. If serious liver injury with clinical symptoms and/or [[hyperbilirubinemia]] or [[jaundice]] occurs during treatment with pravastatin sodium, promptly interrupt therapy. If an alternate etiology is not found do not restart pravastatin sodium. | |||
===Endocrine Function=== | |||
= | [[Statins]] interfere with cholesterol synthesis and lower circulating cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production. Results of clinical trials with pravastatin in males and post-menopausal females were inconsistent with regard to possible effects of the drug on basal steroid hormone levels. In a study of 21 males, the mean testosterone response to human chorionic gonadotropin was significantly reduced (p<0.004) after 16 weeks of treatment with 40 mg of pravastatin. However, the percentage of patients showing a ≥50% rise in plasma testosterone after human chorionic gonadotropin stimulation did not change significantly after therapy in these patients. The effects of statins on spermatogenesis and fertility have not been studied in adequate numbers of patients. The effects, if any, of pravastatin on the pituitary-gonadal axis in pre-menopausal females are unknown. Patients treated with pravastatin who display clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if a [[statin]] or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may diminish the levels or activity of [[steroid hormones]]. | ||
In a placebo-controlled study of 214 pediatric patients with HeFH, of which 106 were treated with pravastatin (20 mg in the children aged 8 to 13 years and 40 mg in the adolescents aged 14 to 18 years) for 2 years, there were no detectable differences seen in any of the endocrine parameters (ACTH, cortisol, DHEAS, FSH, LH, TSH, estradiol [girls] or testosterone [boys]) relative to placebo. There were no detectable differences seen in height and weight changes, testicular volume changes, or Tanner score relative to placebo | |||
|clinicalTrials=Short-Term Controlled Trials | |||
In the pravastatin sodium placebo-controlled clinical trials database of 1313 patients (age range 20 to 76 years, 32.4% women, 93.5% Caucasians, 5% Blacks, 0.9% Hispanics, 0.4% Asians, 0.2% Others) with a median treatment duration of 14 weeks, 3.3% of patients on pravastatin sodium and 1.2% patients on placebo discontinued due to adverse events regardless of causality. The most common adverse reactions that led to treatment discontinuation and occurred at an incidence greater than placebo were: liver function test increased, nausea, anxiety/depression, and dizziness. | |||
All adverse clinical events (regardless of causality) reported in ≥2% of pravastatin-treated patients in placebo-controlled trials of up to 8 months duration are identified in Table 1: | |||
[[File:Pravastatin_adverse_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The safety and tolerability of pravastatin sodium at a dose of 80 mg in 2 controlled trials with a mean exposure of 8.6 months was similar to that of pravastatin sodium at lower doses except that 4 out of 464 patients taking 80 mg of pravastatin had a single elevation of CK >10 times ULN compared to 0 out of 115 patients taking 40 mg of pravastatin. | |||
=== | ===Long-Term Controlled Morbidity and Mortality Trials=== | ||
In the pravastatin sodium placebo-controlled clinical trials database of 21,483 patients (age range 24 to 75 years, 10.3% women, 52.3% Caucasians, 0.8% Blacks, 0.5% Hispanics, 0.1% Asians, 0.1% Others, 46.1% Not Recorded) with a median treatment duration of 261 weeks, 8.1% of patients on pravastatin sodium and 9.3% patients on placebo discontinued due to adverse events regardless of causality. | |||
Adverse event data were pooled from several double-blind, placebo-controlled trials (e.g., West of Scotland Coronary Prevention Study [WOS]; Pravastatin Limitation of Atherosclerosis in the Coronary Arteries study [PLAC I]; Pravastatin, Lipids and Atherosclerosis in the Carotids study [PLAC II]; Regression Growth Evaluation Statin Study [REGRESS]; and Kuopio Atherosclerosis Prevention Study [KAPS]) involving a total of 10,764 patients treated with pravastatin 40 mg and 10,719 patients treated with placebo. The safety and tolerability profile in the pravastatin group was comparable to that of the placebo group. Patients were exposed to pravastatin for a mean of 4.0 to 5.1 years in, among other trials, WOS, and 1.9 to 2.9 years in PLAC I, PLAC II, KAPS, and REGRESS. In these long-term trials, the most common reasons for discontinuation were mild, non-specific gastrointestinal complaints. Collectively, these trials represent 47,613 patient-years of exposure to pravastatin. All clinical adverse events (regardless of causality) occurring in ≥2% of patients treated with pravastatin in these studies are identified in Table 2. | |||
[[File:Pravastatin_adverse_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
In addition to the events listed above in the long-term trials table, events of probable, possible, or uncertain relationship to study drug that occurred in < 2% of pravastatin-treated patients in the long-term trials included the following: | |||
'''Dermatologic''': scalp hair abnormality (including [[alopecia]]), [[urticaria]]. | |||
'''Endocrine/Metabolic''': sexual dysfunction, libido change. | |||
'''General''': [[flushing]]. | |||
'''Immunologic''': [[allergy]], [[edema]] head/neck. | |||
'''Musculoskeletal''': muscle weakness. | |||
'''Nervous System''': [[vertigo]], [[insomnia]], memory impairment, [[neuropathy]] (including [[peripheral neuropathy]]). | |||
'''Special Senses''': taste disturbance. | |||
===Laboratory Test Abnormalities=== | |||
Increases in ALT, AST values and CPK have been observed [see Warnings and Precautions (5.1, 5.2)]. | |||
Transient, asymptomatic [[eosinophilia]] has been reported. Eosinophil counts usually returned to normal despite continued therapy. [[Anemia]], [[thrombocytopenia]], and [[leukopenia]] have been reported with [[statins]]. | |||
===Pediatric Patients=== | |||
In a 2-year, double-blind, placebo-controlled study involving 100 boys and 114 girls with HeFH (n=214; age range 8 to 18.5 years, 53% female, 95% Caucasians, <1% Blacks, 3% Asians, 1% Other), the safety and tolerability profile of pravastatin was generally similar to that of placebo. [See Warnings and Precautions (5.3), Use in Specific Populations (8.4), and Clinical Pharmacology (12.3).] | |||
|postmarketing=In addition to the events reported above, as with other drugs in this class, the following events have been reported rarely during postmarketing experience with pravastatin sodium, regardless of causality assessment: | |||
==== | '''Musculoskeletal''': [[myopathy]], [[rhabdomyolysis]]. | ||
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)]. | |||
'''Nervous System''': [[dysfunction]] of certain cranial nerves (including alteration of taste, impairment of extraocular movement, [[facial paresis]]), [[peripheral nerve palsy]]. | |||
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). | |||
'''[[Hypersensitivity]]''': [[anaphylaxis]], [[angioedema]], [[lupus erythematosus-like syndrome]], [[polymyalgia rheumatica]], [[dermatomyositis]], [[vasculitis]], [[purpura]], [[hemolytic anemia]], positive ANA, ESR increase, [[arthritis]], [[arthralgia]], [[asthenia]], [[photosensitivity]], [[chills]], [[malaise]], [[toxic epidermal necrolysis]], [[erythema multiforme]] (including [[Stevens-Johnson syndrome]]). | |||
'''Gastrointestinal''': abdominal pain, [[constipation]], [[pancreatitis]], [[hepatitis]] (including chronic active hepatitis), [[cholestatic jaundice]], fatty change in liver, [[cirrhosis]], [[fulminant hepatic necrosis]], [[hepatoma]], fatal and non-fatal [[hepatic failure]]. | |||
'''Dermatologic''': a variety of skin changes (e.g., [[nodules]], [[discoloration]], dryness of mucous membranes, changes to hair/nails). | |||
'''Renal''': urinary abnormality (including [[dysuria]], frequency, [[nocturia]]). | |||
'''Respiratory''': [[dyspnea]]. | |||
'''Reproductive''': [[gynecomastia]]. | |||
'''Laboratory Abnormalities''': liver function test abnormalities, thyroid function abnormalities. | |||
|drugInteractions====[[Cyclosporine]]=== | |||
The risk of [[myopathy]]/[[rhabdomyolysis]] is increased with concomitant administration of [[cyclosporine]]. Limit pravastatin to 20 mg once daily for concomitant use with [[cyclosporine]] [see Dosage and Administration (2.5), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)]. | |||
===[[Clarithromycin]]=== | |||
The risk of [[myopathy]]/[[rhabdomyolysis]] is increased with concomitant administration of [[clarithromycin]]. Limit pravastatin to 40 mg once daily for concomitant use with [[clarithromycin]] [see Dosage and Administration (2.6), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)]. | |||
===[[Colchicine]]=== | |||
The risk of [[myopathy]]/[[rhabdomyolysis]] is increased with concomitant administration of [[colchicine]] [see Warnings and Precautions (5.1)]. | |||
=== | ===[[Gemfibrozil]]=== | ||
Due to an increased risk of [[myopathy]]/[[rhabdomyolysis]] when HMG-CoA reductase inhibitors are coadministered with [[gemfibrozil]], concomitant administration of pravastatin sodium with gemfibrozil should be avoided [see Warnings and Precautions (5.1)]. | |||
===Other [[Fibrates]]=== | |||
Because it is known that the risk of [[myopathy]] during treatment with [[HMG-CoA reductase inhibitors]] is increased with concurrent administration of other [[fibrates]], pravastatin sodium should be administered with caution when used concomitantly with other [[fibrates]] [see Warnings and Precautions (5.1)]. | |||
===[[Niacin]]=== | |||
The risk of skeletal muscle effects may be enhanced when pravastatin is used in combination with niacin; a reduction in pravastatin sodium dosage should be considered in this setting [see Warnings and Precautions (5.1)]. | |||
|FDAPregCat=X | |||
|useInPregnancyFDA=Safety in pregnant women has not been established. Available data in women inadvertently taking pravastatin while pregnant do not suggest any adverse clinical events. However, there are no adequate and well-controlled studies in pregnant women. Therefore, it is not known whether pravastatin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Pravastatin should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus and patients have been informed of the potential hazards. | |||
Rare reports of congenital anomalies have been received following intrauterine exposure to other statins. In a review2 of approximately 100 prospectively followed pregnancies in women exposed to simvastatin or lovastatin, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed what would be expected in the general population. The number of cases is adequate to exclude a ≥3- to 4-fold increase in congenital anomalies over the background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. As safety in pregnant women has not been established and there is no apparent benefit to therapy with pravastatin sodium during pregnancy [see Contraindications (4.3)], treatment should be immediately discontinued as soon as pregnancy is recognized. Pravastatin sodium should be administered to women of childbearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards. | |||
Pravastatin was neither embryolethal nor teratogenic in rats at doses up to 1000 mg/kg daily or in rabbits at doses of up to 50 mg/kg daily. These doses resulted in 10 times (rabbit) or 120 times (rat) the human exposure at 80 mg/day maximum recommended human dose (MRHD) based on surface area (mg/m2). | |||
In pregnant rats given oral gavage doses of 4, 20, 100, 500, and 1000 mg/kg/day from gestation days 7 through 17 (organogenesis) increased mortality of offspring and skeletal anomalies were observed at 100 mg/kg/day systemic exposure, 10 times the human exposure at 80 mg/day MRHD based on body surface area (mg/m2). | |||
In pregnant rats given oral gavage doses of 10, 100, and 1000 mg/kg/day from gestation day 17 through lactation day 21 (weaning) increased mortality of offspring and developmental delays were observed at 100 mg/kg/day systemic exposure, 12 times the human exposure at 80 mg/day MRHD based on body surface area (mg/m2). | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=A small amount of pravastatin is excreted in human breast milk. Because of the potential for serious adverse reactions in nursing infants, women taking pravastatin sodium should not nurse [see Contraindications (4.4)]. | |||
Pravastatin crosses the placenta and is found in fetal tissue at 30% maternal plasma levels following a single 20 mg/kg dose given to pregnant rats on gestation day 18. Similar studies in lactating rats indicate secretion of pravastatin into breast milk at 0.2 to 6.5 times higher levels than maternal plasma at exposures equivalent to 2 times human exposure at the MRHD. | |||
|useInPed=The safety and effectiveness of pravastatin sodium in children and adolescents from 8 to 18 years of age have been evaluated in a placebo-controlled study of 2 years duration. Patients treated with pravastatin had an adverse experience profile generally similar to that of patients treated with placebo with influenza and headache commonly reported in both treatment groups. [See Adverse Reactions (6.4).] Doses greater than 40 mg have not been studied in this population. Children and adolescent females of childbearing potential should be counseled on appropriate contraceptive methods while on pravastatin therapy [see Contraindications (4.3) and Use in Specific Populations (8.1)]. For dosing information [see Dosage and Administration (2.3).] | |||
Double-blind, placebo-controlled pravastatin studies in children less than 8 years of age have not been conducted. | |||
|useInGeri=The beneficial effect of pravastatin in elderly subjects in reducing cardiovascular events and in modifying lipid profiles was similar to that seen in younger subjects. The adverse event profile in the elderly was similar to that in the overall population. Other reported clinical experience has not identified differences in responses to pravastatin between elderly and younger patients. | |||
Mean pravastatin AUCs are slightly (25% to 50%) higher in elderly subjects than in healthy young subjects, but mean maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), and half-life (t½) values are similar in both age groups and substantial accumulation of pravastatin would not be expected in the elderly [see Clinical Pharmacology (12.3)]. | |||
Since advanced age (≥65 years) is a predisposing factor for myopathy, pravastatin sodium should be prescribed with caution in the elderly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
| | |othersTitle=Homozygous Familial Hypercholesterolemia | ||
|useInOthers=Pravastatin has not been evaluated in patients with rare homozygous familial hypercholesterolemia. In this group of patients, it has been reported that statins are less effective because the patients lack functional LDL receptors. | |||
|administration=* Oral | |||
| | |monitoring=FDA Package Insert for Pravastatin contains no information regarding drug monitoring. | ||
There is | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
| | |overdose=To date, there has been limited experience with overdosage of pravastatin. If an overdose occurs, it should be treated symptomatically with laboratory monitoring and supportive measures should be instituted as required. | ||
|drugBox={{Drugbox | |||
| verifiedrevid = 464213376 | |||
| IUPAC_name = (3''R'',5''R'')-3,5-dihydroxy-7-((1''R'',2''S'',6''S'',8''R'',8a''R'')-6-hydroxy-2-methyl-8-{[(2''S'')-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)-heptanoic acid | |||
| image = Pravastatin.PNG | |||
| width = 181 | |||
| | <!--Clinical data--> | ||
| tradename = Pravachol | |||
| Drugs.com = {{drugs.com|monograph|pravachol}} | |||
| MedlinePlus = a692025 | |||
| pregnancy_AU = D | |||
| pregnancy_US = X | |||
| legal_AU = S4 | |||
| legal_CA = Rx-only | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = oral | |||
| | <!--Pharmacokinetic data--> | ||
| bioavailability = 18%<ref name = PK>{{cite journal|last=Neuvonen|first=PJ|author2=Backman, JT |author3=Niemi, M |title=Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin.|journal=Clinical Pharmacokinetics|date=2008|volume=47|issue=7|pages=463-74|doi=10.2165/00003088-200847070-00003|pmid=18563955}}</ref> | |||
| protein_bound = 50%<ref name = PK/> | |||
| metabolism = Hepatic (minimal; [[CYP3A4]]-mediated)<ref name = PK/> | |||
| elimination_half-life = 1-3 hours<ref name = PK/> | |||
| | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| | | CAS_number = 81093-37-0 | ||
| ATC_prefix = C10 | |||
| ATC_suffix = AA03 | |||
| | | ATC_supplemental = | ||
| PubChem = 54687 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00175 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | | ChemSpiderID = 49398 | ||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = KXO2KT9N0G | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1144 | |||
| | |||
| | |||
| | |||
| | |||
<!--Chemical data--> | |||
| C=23 | H=36 | O=7 | |||
| molecular_weight = 424.528 g/mol | |||
| smiles = O=C(O)C[C@H](O)C[C@H](O)CC[C@H]2[C@H](/C=C\C1=C\[C@@H](O)C[C@H](OC(=O)[C@@H](C)CC)[C@@H]12)C | |||
| InChI = 1/C23H36O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,9,13-14,16-20,22,24-26H,4,7-8,10-12H2,1-3H3,(H,27,28)/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | |||
| InChIKey = TUZYXOIXSAXUGO-PZAWKZKUBI | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C23H36O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,9,13-14,16-20,22,24-26H,4,7-8,10-12H2,1-3H3,(H,27,28)/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = TUZYXOIXSAXUGO-PZAWKZKUSA-N | |||
}} | |||
|mechAction=Pravastatin is a reversible inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in the biosynthetic pathway for cholesterol. In addition, pravastatin reduces VLDL and TG and increases HDL-C. | |||
|structure=Pravastatin sodium tablets USP are one of a class of lipid-lowering compounds, the statins, which reduce cholesterol biosynthesis. These agents are competitive inhibitors of HMG-CoA reductase, the enzyme catalyzing the early rate-limiting step in cholesterol biosynthesis, conversion of HMG-CoA to mevalonate. | |||
Pravastatin sodium USP is designated chemically as 1-Naphthalene-heptanoic acid, 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, monosodium salt, [1S[1α(βS*,δS*),2α,6α,8β(R*),8aα]]-. | |||
Structural formula: | |||
[[File:Pravastatin_strcture_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
C23H35NaO7 MW 446.52 | |||
Pravastatin sodium USP is an odorless, white to yellowish white, hygroscpic powder. It is a relatively polar hydrophilic compound with a partition coefficient (octanol/water) of 0.59 at a pH of 7. It is freely soluble in water and methanol, soluble in alcohol, very slightly soluble in acetonitrile and practically insoluble in ether, ethyl acetate and chloroform. | |||
|PD=====General==== | |||
'''Absorption''': Pravastatin sodium is administered orally in the active form. In studies in man, peak plasma pravastatin concentrations occurred 1 to 1.5 hours upon oral administration. Based on urinary recovery of total radiolabeled drug, the average oral absorption of pravastatin is 34% and absolute bioavailability is 17%. While the presence of food in the gastrointestinal tract reduces systemic bioavailability, the lipid-lowering effects of the drug are similar whether taken with or 1 hour prior to meals. | |||
Pravastatin plasma concentrations, including area under the concentration-time curve (AUC), Cmax, and steady-state minimum (Cmin), are directly proportional to administered dose. Systemic bioavailability of pravastatin administered following a bedtime dose was decreased 60% compared to that following an AM dose. Despite this decrease in systemic bioavailability, the efficacy of pravastatin administered once daily in the evening, although not statistically significant, was marginally more effective than that after a morning dose. | |||
The coefficient of variation (CV), based on between-subject variability, was 50% to 60% for AUC. The geometric means of pravastatin Cmax and AUC following a 20 mg dose in the fasted state were 26.5 ng/mL and 59.8 ng*hr/mL, respectively. | |||
Steady-state AUCs, Cmax, and Cmin plasma concentrations showed no evidence of pravastatin accumulation following once or twice daily administration of pravastatin sodium tablets. | |||
'''Distribution''': Approximately 50% of the circulating drug is bound to plasma proteins. | |||
'''Metabolism''': The major biotransformation pathways for pravastatin are: (a) isomerization to 6-epi pravastatin and the 3α-hydroxyisomer of pravastatin (SQ 31,906) and (b) enzymatic ring hydroxylation to SQ 31,945. The 3α-hydroxyisomeric metabolite (SQ 31,906) has 1/10 to 1/40 the HMG-CoA reductase inhibitory activity of the parent compound. Pravastatin undergoes extensive first-pass extraction in the liver (extraction ratio 0.66). | |||
'''Excretion''': Approximately 20% of a radiolabeled oral dose is excreted in urine and 70% in the feces. After intravenous administration of radiolabeled pravastatin to normal volunteers, approximately 47% of total body clearance was via renal excretion and 53% by non-renal routes (i.e., biliary excretion and biotransformation). | |||
Following single dose oral administration of 14C-pravastatin, the radioactive elimination t½ for pravastatin is 1.8 hours in humans. | |||
====Specific Populations==== | |||
* | '''Renal Impairment''': A single 20 mg oral dose of pravastatin was administered to 24 patients with varying degrees of renal impairment (as determined by creatinine clearance). No effect was observed on the pharmacokinetics of pravastatin or its 3α-hydroxy isomeric metabolite (SQ 31,906). Compared to healthy subjects with normal renal function, patients with severe renal impairment had 69% and 37% higher mean AUC and Cmax values, respectively, and a 0.61 hour shorter t½ for the inactive enzymatic ring hydroxylation metabolite (SQ 31,945). | ||
'''Hepatic Impairment''': In a study comparing the kinetics of pravastatin in patients with biopsy confirmed cirrhosis (N=7) and normal subjects (N=7), the mean AUC varied 18-fold in cirrhotic patients and 5-fold in healthy subjects. Similarly, the peak pravastatin values varied 47-fold for cirrhotic patients compared to 6-fold for healthy subjects. [See Warnings and Precautions (5.2).] | |||
'''Geriatric''': In a single oral dose study using pravastatin 20 mg, the mean AUC for pravastatin was approximately 27% greater and the mean cumulative urinary excretion (CUE) approximately 19% lower in elderly men (65 to 75 years old) compared with younger men (19 to 31 years old). In a similar study conducted in women, the mean AUC for pravastatin was approximately 46% higher and the mean CUE approximately 18% lower in elderly women (65 to 78 years old) compared with younger women (18 to 38 years old). In both studies, Cmax, Tmax, and t½ values were similar in older and younger subjects. [See Use in Specific Populations (8.5).] | |||
'''Pediatric''': After 2 weeks of once-daily 20 mg oral pravastatin administration, the geometric means of AUC were 80.7 (CV 44%) and 44.8 (CV 89%) ng*hr/mL for children (8 to 11 years, N=14) and adolescents (12 to 16 years, N=10), respectively. The corresponding values for Cmax were 42.4 (CV 54%) and 18.6 ng/mL (CV 100%) for children and adolescents, respectively. No conclusion can be made based on these findings due to the small number of samples and large variability. [See Use in Specific Populations (8.4).] | |||
====Drug-Drug Interactions==== | |||
[[File:Pravastatin_PD_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
| | [[File:Pravastatin_PD_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|PK= | |PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | ||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic====Carcinogenesis, Mutagenesis, Impairment of Fertility=== | |||
In a 2-year study in rats fed pravastatin at doses of 10, 30, or 100 mg/kg body weight, there was an increased incidence of hepatocellular carcinomas in males at the highest dose (p<0.01). These effects in rats were observed at approximately 12 times the human dose (HD) of 80 mg based on body surface area (mg/m2) and at approximately 4 times the HD, based on AUC. | |||
In a 2-year study in mice fed pravastatin at doses of 250 and 500 mg/kg/day, there was an increased incidence of hepatocellular carcinomas in males and females at both 250 and 500 mg/kg/day (p<0.0001). At these doses, lung adenomas in females were increased (p=0.013). These effects in mice were observed at approximately 15 times (250 mg/kg/day) and 23 times (500 mg/kg/day) the HD of 80 mg, based on AUC. In another 2-year study in mice with doses up to 100 mg/kg/day (producing drug exposures approximately 2 times the HD of 80 mg, based on AUC), there were no drug-induced tumors. | |||
No evidence of mutagenicity was observed in vitro, with or without rat-liver metabolic activation, in the following studies: microbial mutagen tests, using mutant strains of Salmonella typhimurium or Escherichia coli; a forward mutation assay in L5178Y TK +/− mouse lymphoma cells; a chromosomal aberration test in hamster cells; and a gene conversion assay using Saccharomyces cerevisiae. In addition, there was no evidence of mutagenicity in either a dominant lethal test in mice or a micronucleus test in mice. | |||
In a fertility study in adult rats with daily doses up to 500 mg/kg, pravastatin did not produce any adverse effects on fertility or general reproductive performance. | |||
===Animal Toxicology and/or Pharmacology=== | |||
====CNS Toxicity==== | |||
|clinicalStudies= | CNS vascular lesions, characterized by perivascular hemorrhage and edema and mononuclear cell infiltration of perivascular spaces, were seen in dogs treated with pravastatin at a dose of 25 mg/kg/day. These effects in dogs were observed at approximately 59 times the HD of 80 mg/day, based on AUC. Similar CNS vascular lesions have been observed with several other drugs in this class. | ||
A chemically similar drug in this class produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). This same drug also produced vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis in dogs treated for 14 weeks at 180 mg/kg/day, a dose which resulted in a mean plasma drug level similar to that seen with the 60 mg/kg/day dose. | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | When administered to juvenile rats (postnatal days [PND] 4 through 80 at 5 to 45 mg/kg/day), no drug related changes were observed at 5 mg/kg/day. At 15 and 45 mg/kg/day, altered body-weight gain was observed during the dosing and 52-day recovery periods as well as slight thinning of the corpus callosum at the end of the recovery period. This finding was not evident in rats examined at the completion of the dosing period and was not associated with any inflammatory or degenerative changes in the brain. The biological relevance of the corpus callosum finding is uncertain due to the absence of any other microscopic changes in the brain or peripheral nervous tissue and because it occurred at the end of the recovery period. Neurobehavioral changes (enhanced acoustic startle responses and increased errors in water-maze learning) combined with evidence of generalized toxicity were noted at 45 mg/kg/day during the later part of the recovery period. Serum pravastatin levels at 15 mg/kg/day are approximately ≥1 times (AUC) the maximum pediatric dose of 40 mg. No thinning of the corpus callosum was observed in rats dosed with pravastatin (≥250 mg/kg/day) beginning PND 35 for 3 months suggesting increased sensitivity in younger rats. PND 35 in a rat is approximately equivalent to an 8- to 12-year-old human child. Juvenile male rats given 90 times (AUC) the 40 mg dose had decreased fertility (20%) with sperm abnormalities compared to controls. | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
=====Condition1===== | =====Condition1===== | ||
| Line 441: | Line 375: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* ®<ref>{{Cite web | title = | url = }}</ref> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage | |||
|fileName=No image.jpg | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
Revision as of 20:48, 25 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pravastatin is a HMG-COA Reductase Inhibitor that is FDA approved for the treatment of hypercholesterolemia, prevention of cardiovascular disease. Common adverse reactions include Rash, Diarrhea, Nausea and vomiting, Musculoskeletal pain, Headache, Cough, Rhinitis, Upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypercholesterolemia
- Indication
- Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate.
Prevention of Cardiovascular Disease
- Indication
- In hypercholesterolemic patients without clinically evident coronary heart disease (CHD), pravastatin sodium tablets USP are indicated to:
- reduce the risk of myocardial infarction (MI).
- reduce the risk of undergoing myocardial revascularization procedures.
- reduce the risk of cardiovascular mortality with no increase in death from non-cardiovascular causes.
- General Dosing Information
- The patient should be placed on a standard cholesterol-lowering diet before receiving pravastatin sodium tablets and should continue on this diet during treatment with pravastatin sodium tablets [see NCEP Treatment Guidelines for details on dietary therapy].
- Recommended starting dosage: 40 mg PO qd.
- If a daily dose of 40 mg does not achieve desired cholesterol levels, 80 mg once daily is recommended.
- In patients with significant renal impairment,
- Starting dosage: 10 mg PO qd. Pravastatin sodium tablets can be administered orally as a single dose at any time of the day, with or without food.
- Since the maximal effect of a given dose is seen within 4 weeks, periodic lipid determinations should be performed at this time and dosage adjusted according to the patient’s response to therapy and established treatment guidelines.
Concomitant Lipid-Altering Therapy
- Dosing information
- Pravastatin sodium tablets may be used with bile acid resins. When administering a bile-acid-binding resin (e.g., cholestyramine, colestipol) and pravastatin, pravastatin sodium tablets should be given either 1 hour or more before or at least 4 hours following the resin. [See Clinical Pharmacology (12.3).]
Dosage in Patients Taking Cyclosporine
- Dosing information
- In patients taking immunosuppressive drugs such as cyclosporine concomitantly with pravastatin, therapy should begin with 10 mg of pravastatin sodium once-a-day at bedtime and titration to higher doses should be done with caution.
- Most patients treated with this combination received a maximum pravastatin sodium dose of 20 mg/day.
- In patients taking cyclosporine: less than 20 mg PO qd [see Warnings and Precautions (5.1) and Drug Interactions (7.1)].
Dosage in Patients Taking Clarithromycin
- Dosing information
- In patients taking clarithromycin: less than 40 mg PO qd [see Drug Interactions (7.2)].
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pravastatin in adult patients.
Non–Guideline-Supported Use
Acute coronary syndrome
- Dosing information
Atrial fibrillation
- Dosing information
- 80 mg/day18242276
Coronary artery bypass graft
- Dosing information
- 10-20 mg.day 11074212
Heart failure
- Dosing information
- HMG-CoA-reductase inhibitor (statin) therapy reduced the risks of all-cause mortality and hospitalization for heart failure among patients with chronic heart failure11074212
Kidney disease
- Dosing information
- 10 mg/day12105140
Nephrotic syndrome
- Dosing information
- 20 mg PO bid10644862
Percutaneous coronary intervention
- Dosing information
- 40 mg/day8840836
Radiographic contrast agent nephropathy
- Dosing information
- 10 mg/day 19782255
Restenotic lesion of coronary artery
- Dosing information
- 40 mg PO qd 11018193
Transient ischemic attack
- Dosing information
- 20 mg/day 11222801
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hypercholesterolemia
- Dosing information
- Children (Ages 8 to 13 Years, Inclusive)
- Recommended dose: 20 mg PO qd in children 8 to 13 years of age. Doses greater than 20 mg have not been studied in this patient population.
- Adolescents (Ages 14 to 18 Years)
- Recommended starting dose: 40 mg PO qd in adolescents 14 to 18 years of age. Doses greater than 40 mg have not been studied in this patient population.
- Children and adolescents treated with pravastatin should be reevaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult goals for LDL-C [see Indications and Usage (1.2)].
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pravastatin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pravastatin in pediatric patients.
Contraindications
Hypersensitivity
Hypersensitivity to any component of this medication.
Liver
Active liver disease or unexplained, persistent elevations of serum transaminases [see Warnings and Precautions (5.2)].
Pregnancy
Atherosclerosis is a chronic process and discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. cholesterol and other products of cholesterol biosynthesis are essential components for fetal development (including synthesis of steroids and cell membranes). Since statins decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, they are contraindicated during pregnancy and in nursing mothers. PRAVASTATIN SHOULD BE ADMINISTERED TO WOMEN OF CHILDBEARING AGE ONLY WHEN SUCH PATIENTS ARE HIGHLY UNLIKELY TO CONCEIVE AND HAVE BEEN INFORMED OF THE POTENTIAL HAZARDS. If the patient becomes pregnant while taking this class of drug, therapy should be discontinued immediately and the patient apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
Nursing Mothers
A small amount of pravastatin is excreted in human breast milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require pravastatin sodium treatment should not breast-feed their infants [see Use in Specific Populations (8.3)].
Warnings
Skeletal Muscle
Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with pravastatin and other drugs in this class. A history of renal impairment may be a risk factor for the development of rhabdomyolysis. Such patients merit closer monitoring for skeletal muscle effects. Uncomplicated myalgia has also been reported in pravastatin-treated patients [see Adverse Reactions (6)]. Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values to greater than 10 times the upper limit of normal (ULN), was rare (<0.1%) in pravastatin clinical trials. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK. Predisposing factors include advanced age (>65), uncontrolled hypothyroidism, and renal impairment. There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum CPK, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation and improvement with immunosuppressive agents. All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing pravastatin sodium. Pravastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Pravastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy. The risk of myopathy during treatment with statins is increased with concurrent therapy with either erythromycin, cyclosporine, niacin, or fibrates. However, neither myopathy nor significant increases in CPK levels have been observed in 3 reports involving a total of 100 post-transplant patients (24 renal and 76 cardiac) treated for up to 2 years concurrently with pravastatin 10 to 40 mg and cyclosporine. Some of these patients also received other concomitant immunosuppressive therapies. Further, in clinical trials involving small numbers of patients who were treated concurrently with pravastatin and niacin, there were no reports of myopathy. Also, myopathy was not reported in a trial of combination pravastatin (40 mg/day) and gemfibrozil (1200 mg/day), although 4 of 75 patients on the combination showed marked CPK elevations versus 1 of 73 patients receiving placebo. There was a trend toward more frequent CPK elevations and patient withdrawals due to musculoskeletal symptoms in the group receiving combined treatment as compared with the groups receiving placebo, gemfibrozil, or pravastatin monotherapy. The use of fibrates alone may occasionally be associated with myopathy. The benefit of further alterations in lipid levels by the combined use of pravastatin sodiumwith fibrates should be carefully weighed against the potential risks of this combination. Cases of myopathy, including rhabdomyolysis, have been reported with pravastatin coadministered with colchicine, and caution should be exercised when prescribing pravastatin with colchicine [see Drug Interactions (7.3)].
Liver
Statins, like some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. In placebo-controlled clinical trials subjects were exposed to pravastatin or placebo [see Clinical Studies (14)]. In an analysis of serum transaminase values (ALT, AST), incidences of marked abnormalities were compared between the pravastatin and placebo treatment groups; a marked abnormality was defined as a post-treatment test value greater than 3 times the upper limit of normal for subjects with pretreatment values less than or equal to the upper limit of normal, or 4 times the pretreatment value for subjects with pretreatment values greater than the upper limit of normal but less than 1.5 times the upper limit of normal. Marked abnormalities of ALT or AST occurred with similar low frequency (≤1.2%) in both treatment groups. Overall, clinical trial experience showed that liver function test abnormalities observed during pravastatin therapy were usually asymptomatic, not associated with cholestasis, and did not appear to be related to treatment duration. In a 320-patient placebo-controlled clinical trial, subjects with chronic (>6 months) stable liver disease, due primarily to hepatitis C or nonalcoholic fatty liver disease, were treated with 80 mg pravastatin or placebo for up to 9 months. The primary safety endpoint was the proportion of subjects with at least one ALT ≥2 times the upper limit of normal for those with normal ALT (≤ the upper limit of normal) at baseline or a doubling of the baseline ALT for those with elevated ALT (> the upper limit of normal) at baseline. By Week 36, 12 out of 160 (7.5%) subjects treated with pravastatin met the prespecified safety ALT endpoint compared to 20 out of 160 (12.5%) subjects receiving placebo. Conclusions regarding liver safety are limited since the study was not large enough to establish similarity between groups (with 95% confidence) in the rates of ALT elevation. It is recommended that liver function tests be performed prior to the initiation of therapy and when clinically indicated. Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of pravastatin [see Contraindications (4.2)]. Caution should be exercised when pravastatin is administered to patients who have a recent (<6 months) history of liver disease, have signs that may suggest liver disease (e.g., unexplained aminotransferase elevations, jaundice), or are heavy users of alcohol. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including pravastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with pravastatin sodium, promptly interrupt therapy. If an alternate etiology is not found do not restart pravastatin sodium.
Endocrine Function
Statins interfere with cholesterol synthesis and lower circulating cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production. Results of clinical trials with pravastatin in males and post-menopausal females were inconsistent with regard to possible effects of the drug on basal steroid hormone levels. In a study of 21 males, the mean testosterone response to human chorionic gonadotropin was significantly reduced (p<0.004) after 16 weeks of treatment with 40 mg of pravastatin. However, the percentage of patients showing a ≥50% rise in plasma testosterone after human chorionic gonadotropin stimulation did not change significantly after therapy in these patients. The effects of statins on spermatogenesis and fertility have not been studied in adequate numbers of patients. The effects, if any, of pravastatin on the pituitary-gonadal axis in pre-menopausal females are unknown. Patients treated with pravastatin who display clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if a statin or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may diminish the levels or activity of steroid hormones. In a placebo-controlled study of 214 pediatric patients with HeFH, of which 106 were treated with pravastatin (20 mg in the children aged 8 to 13 years and 40 mg in the adolescents aged 14 to 18 years) for 2 years, there were no detectable differences seen in any of the endocrine parameters (ACTH, cortisol, DHEAS, FSH, LH, TSH, estradiol [girls] or testosterone [boys]) relative to placebo. There were no detectable differences seen in height and weight changes, testicular volume changes, or Tanner score relative to placebo
Adverse Reactions
Clinical Trials Experience
Short-Term Controlled Trials In the pravastatin sodium placebo-controlled clinical trials database of 1313 patients (age range 20 to 76 years, 32.4% women, 93.5% Caucasians, 5% Blacks, 0.9% Hispanics, 0.4% Asians, 0.2% Others) with a median treatment duration of 14 weeks, 3.3% of patients on pravastatin sodium and 1.2% patients on placebo discontinued due to adverse events regardless of causality. The most common adverse reactions that led to treatment discontinuation and occurred at an incidence greater than placebo were: liver function test increased, nausea, anxiety/depression, and dizziness. All adverse clinical events (regardless of causality) reported in ≥2% of pravastatin-treated patients in placebo-controlled trials of up to 8 months duration are identified in Table 1:

The safety and tolerability of pravastatin sodium at a dose of 80 mg in 2 controlled trials with a mean exposure of 8.6 months was similar to that of pravastatin sodium at lower doses except that 4 out of 464 patients taking 80 mg of pravastatin had a single elevation of CK >10 times ULN compared to 0 out of 115 patients taking 40 mg of pravastatin.
Long-Term Controlled Morbidity and Mortality Trials
In the pravastatin sodium placebo-controlled clinical trials database of 21,483 patients (age range 24 to 75 years, 10.3% women, 52.3% Caucasians, 0.8% Blacks, 0.5% Hispanics, 0.1% Asians, 0.1% Others, 46.1% Not Recorded) with a median treatment duration of 261 weeks, 8.1% of patients on pravastatin sodium and 9.3% patients on placebo discontinued due to adverse events regardless of causality. Adverse event data were pooled from several double-blind, placebo-controlled trials (e.g., West of Scotland Coronary Prevention Study [WOS]; Pravastatin Limitation of Atherosclerosis in the Coronary Arteries study [PLAC I]; Pravastatin, Lipids and Atherosclerosis in the Carotids study [PLAC II]; Regression Growth Evaluation Statin Study [REGRESS]; and Kuopio Atherosclerosis Prevention Study [KAPS]) involving a total of 10,764 patients treated with pravastatin 40 mg and 10,719 patients treated with placebo. The safety and tolerability profile in the pravastatin group was comparable to that of the placebo group. Patients were exposed to pravastatin for a mean of 4.0 to 5.1 years in, among other trials, WOS, and 1.9 to 2.9 years in PLAC I, PLAC II, KAPS, and REGRESS. In these long-term trials, the most common reasons for discontinuation were mild, non-specific gastrointestinal complaints. Collectively, these trials represent 47,613 patient-years of exposure to pravastatin. All clinical adverse events (regardless of causality) occurring in ≥2% of patients treated with pravastatin in these studies are identified in Table 2.

In addition to the events listed above in the long-term trials table, events of probable, possible, or uncertain relationship to study drug that occurred in < 2% of pravastatin-treated patients in the long-term trials included the following:
Dermatologic: scalp hair abnormality (including alopecia), urticaria. Endocrine/Metabolic: sexual dysfunction, libido change. General: flushing. Immunologic: allergy, edema head/neck. Musculoskeletal: muscle weakness. Nervous System: vertigo, insomnia, memory impairment, neuropathy (including peripheral neuropathy). Special Senses: taste disturbance.
Laboratory Test Abnormalities
Increases in ALT, AST values and CPK have been observed [see Warnings and Precautions (5.1, 5.2)]. Transient, asymptomatic eosinophilia has been reported. Eosinophil counts usually returned to normal despite continued therapy. Anemia, thrombocytopenia, and leukopenia have been reported with statins.
Pediatric Patients
In a 2-year, double-blind, placebo-controlled study involving 100 boys and 114 girls with HeFH (n=214; age range 8 to 18.5 years, 53% female, 95% Caucasians, <1% Blacks, 3% Asians, 1% Other), the safety and tolerability profile of pravastatin was generally similar to that of placebo. [See Warnings and Precautions (5.3), Use in Specific Populations (8.4), and Clinical Pharmacology (12.3).]
Postmarketing Experience
In addition to the events reported above, as with other drugs in this class, the following events have been reported rarely during postmarketing experience with pravastatin sodium, regardless of causality assessment:
Musculoskeletal: myopathy, rhabdomyolysis. There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)]. Nervous System: dysfunction of certain cranial nerves (including alteration of taste, impairment of extraocular movement, facial paresis), peripheral nerve palsy. There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). Hypersensitivity: anaphylaxis, angioedema, lupus erythematosus-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, hemolytic anemia, positive ANA, ESR increase, arthritis, arthralgia, asthenia, photosensitivity, chills, malaise, toxic epidermal necrolysis, erythema multiforme (including Stevens-Johnson syndrome). Gastrointestinal: abdominal pain, constipation, pancreatitis, hepatitis (including chronic active hepatitis), cholestatic jaundice, fatty change in liver, cirrhosis, fulminant hepatic necrosis, hepatoma, fatal and non-fatal hepatic failure. Dermatologic: a variety of skin changes (e.g., nodules, discoloration, dryness of mucous membranes, changes to hair/nails). Renal: urinary abnormality (including dysuria, frequency, nocturia). Respiratory: dyspnea. Reproductive: gynecomastia. Laboratory Abnormalities: liver function test abnormalities, thyroid function abnormalities.
Drug Interactions
Cyclosporine
The risk of myopathy/rhabdomyolysis is increased with concomitant administration of cyclosporine. Limit pravastatin to 20 mg once daily for concomitant use with cyclosporine [see Dosage and Administration (2.5), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
Clarithromycin
The risk of myopathy/rhabdomyolysis is increased with concomitant administration of clarithromycin. Limit pravastatin to 40 mg once daily for concomitant use with clarithromycin [see Dosage and Administration (2.6), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
Colchicine
The risk of myopathy/rhabdomyolysis is increased with concomitant administration of colchicine [see Warnings and Precautions (5.1)].
Gemfibrozil
Due to an increased risk of myopathy/rhabdomyolysis when HMG-CoA reductase inhibitors are coadministered with gemfibrozil, concomitant administration of pravastatin sodium with gemfibrozil should be avoided [see Warnings and Precautions (5.1)].
Other Fibrates
Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concurrent administration of other fibrates, pravastatin sodium should be administered with caution when used concomitantly with other fibrates [see Warnings and Precautions (5.1)].
Niacin
The risk of skeletal muscle effects may be enhanced when pravastatin is used in combination with niacin; a reduction in pravastatin sodium dosage should be considered in this setting [see Warnings and Precautions (5.1)].
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X
Safety in pregnant women has not been established. Available data in women inadvertently taking pravastatin while pregnant do not suggest any adverse clinical events. However, there are no adequate and well-controlled studies in pregnant women. Therefore, it is not known whether pravastatin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Pravastatin should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus and patients have been informed of the potential hazards.
Rare reports of congenital anomalies have been received following intrauterine exposure to other statins. In a review2 of approximately 100 prospectively followed pregnancies in women exposed to simvastatin or lovastatin, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed what would be expected in the general population. The number of cases is adequate to exclude a ≥3- to 4-fold increase in congenital anomalies over the background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. As safety in pregnant women has not been established and there is no apparent benefit to therapy with pravastatin sodium during pregnancy [see Contraindications (4.3)], treatment should be immediately discontinued as soon as pregnancy is recognized. Pravastatin sodium should be administered to women of childbearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards.
Pravastatin was neither embryolethal nor teratogenic in rats at doses up to 1000 mg/kg daily or in rabbits at doses of up to 50 mg/kg daily. These doses resulted in 10 times (rabbit) or 120 times (rat) the human exposure at 80 mg/day maximum recommended human dose (MRHD) based on surface area (mg/m2).
In pregnant rats given oral gavage doses of 4, 20, 100, 500, and 1000 mg/kg/day from gestation days 7 through 17 (organogenesis) increased mortality of offspring and skeletal anomalies were observed at 100 mg/kg/day systemic exposure, 10 times the human exposure at 80 mg/day MRHD based on body surface area (mg/m2).
In pregnant rats given oral gavage doses of 10, 100, and 1000 mg/kg/day from gestation day 17 through lactation day 21 (weaning) increased mortality of offspring and developmental delays were observed at 100 mg/kg/day systemic exposure, 12 times the human exposure at 80 mg/day MRHD based on body surface area (mg/m2).
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pravastatin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pravastatin during labor and delivery.
Nursing Mothers
A small amount of pravastatin is excreted in human breast milk. Because of the potential for serious adverse reactions in nursing infants, women taking pravastatin sodium should not nurse [see Contraindications (4.4)]. Pravastatin crosses the placenta and is found in fetal tissue at 30% maternal plasma levels following a single 20 mg/kg dose given to pregnant rats on gestation day 18. Similar studies in lactating rats indicate secretion of pravastatin into breast milk at 0.2 to 6.5 times higher levels than maternal plasma at exposures equivalent to 2 times human exposure at the MRHD.
Pediatric Use
The safety and effectiveness of pravastatin sodium in children and adolescents from 8 to 18 years of age have been evaluated in a placebo-controlled study of 2 years duration. Patients treated with pravastatin had an adverse experience profile generally similar to that of patients treated with placebo with influenza and headache commonly reported in both treatment groups. [See Adverse Reactions (6.4).] Doses greater than 40 mg have not been studied in this population. Children and adolescent females of childbearing potential should be counseled on appropriate contraceptive methods while on pravastatin therapy [see Contraindications (4.3) and Use in Specific Populations (8.1)]. For dosing information [see Dosage and Administration (2.3).] Double-blind, placebo-controlled pravastatin studies in children less than 8 years of age have not been conducted.
Geriatic Use
The beneficial effect of pravastatin in elderly subjects in reducing cardiovascular events and in modifying lipid profiles was similar to that seen in younger subjects. The adverse event profile in the elderly was similar to that in the overall population. Other reported clinical experience has not identified differences in responses to pravastatin between elderly and younger patients. Mean pravastatin AUCs are slightly (25% to 50%) higher in elderly subjects than in healthy young subjects, but mean maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), and half-life (t½) values are similar in both age groups and substantial accumulation of pravastatin would not be expected in the elderly [see Clinical Pharmacology (12.3)]. Since advanced age (≥65 years) is a predisposing factor for myopathy, pravastatin sodium should be prescribed with caution in the elderly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
Gender
There is no FDA guidance on the use of Pravastatin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pravastatin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pravastatin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pravastatin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pravastatin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pravastatin in patients who are immunocompromised.
Homozygous Familial Hypercholesterolemia
Pravastatin has not been evaluated in patients with rare homozygous familial hypercholesterolemia. In this group of patients, it has been reported that statins are less effective because the patients lack functional LDL receptors.
Administration and Monitoring
Administration
- Oral
Monitoring
FDA Package Insert for Pravastatin contains no information regarding drug monitoring.
IV Compatibility
There is limited information regarding IV Compatibility of Pravastatin in the drug label.
Overdosage
To date, there has been limited experience with overdosage of pravastatin. If an overdose occurs, it should be treated symptomatically with laboratory monitoring and supportive measures should be instituted as required.
Pharmacology
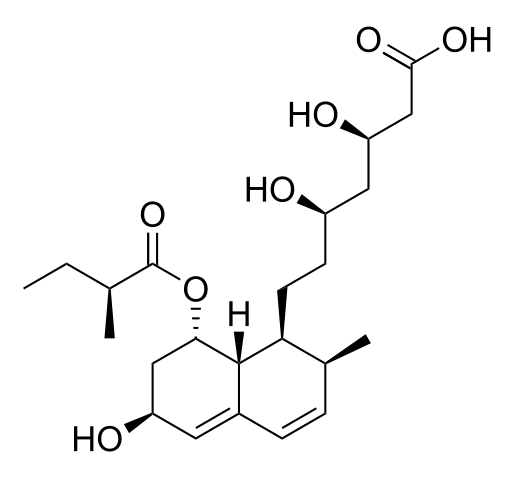 | |
| Clinical data | |
|---|---|
| Trade names | Pravachol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692025 |
| Pregnancy category | |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 18%[1] |
| Protein binding | 50%[1] |
| Metabolism | Hepatic (minimal; CYP3A4-mediated)[1] |
| Elimination half-life | 1-3 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H36O7 |
| Molar mass | 424.528 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
Pravastatin is a reversible inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in the biosynthetic pathway for cholesterol. In addition, pravastatin reduces VLDL and TG and increases HDL-C.
Structure
Pravastatin sodium tablets USP are one of a class of lipid-lowering compounds, the statins, which reduce cholesterol biosynthesis. These agents are competitive inhibitors of HMG-CoA reductase, the enzyme catalyzing the early rate-limiting step in cholesterol biosynthesis, conversion of HMG-CoA to mevalonate. Pravastatin sodium USP is designated chemically as 1-Naphthalene-heptanoic acid, 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, monosodium salt, [1S[1α(βS*,δS*),2α,6α,8β(R*),8aα]]-. Structural formula:

C23H35NaO7 MW 446.52
Pravastatin sodium USP is an odorless, white to yellowish white, hygroscpic powder. It is a relatively polar hydrophilic compound with a partition coefficient (octanol/water) of 0.59 at a pH of 7. It is freely soluble in water and methanol, soluble in alcohol, very slightly soluble in acetonitrile and practically insoluble in ether, ethyl acetate and chloroform.
Pharmacodynamics
General
Absorption: Pravastatin sodium is administered orally in the active form. In studies in man, peak plasma pravastatin concentrations occurred 1 to 1.5 hours upon oral administration. Based on urinary recovery of total radiolabeled drug, the average oral absorption of pravastatin is 34% and absolute bioavailability is 17%. While the presence of food in the gastrointestinal tract reduces systemic bioavailability, the lipid-lowering effects of the drug are similar whether taken with or 1 hour prior to meals. Pravastatin plasma concentrations, including area under the concentration-time curve (AUC), Cmax, and steady-state minimum (Cmin), are directly proportional to administered dose. Systemic bioavailability of pravastatin administered following a bedtime dose was decreased 60% compared to that following an AM dose. Despite this decrease in systemic bioavailability, the efficacy of pravastatin administered once daily in the evening, although not statistically significant, was marginally more effective than that after a morning dose. The coefficient of variation (CV), based on between-subject variability, was 50% to 60% for AUC. The geometric means of pravastatin Cmax and AUC following a 20 mg dose in the fasted state were 26.5 ng/mL and 59.8 ng*hr/mL, respectively. Steady-state AUCs, Cmax, and Cmin plasma concentrations showed no evidence of pravastatin accumulation following once or twice daily administration of pravastatin sodium tablets. Distribution: Approximately 50% of the circulating drug is bound to plasma proteins. Metabolism: The major biotransformation pathways for pravastatin are: (a) isomerization to 6-epi pravastatin and the 3α-hydroxyisomer of pravastatin (SQ 31,906) and (b) enzymatic ring hydroxylation to SQ 31,945. The 3α-hydroxyisomeric metabolite (SQ 31,906) has 1/10 to 1/40 the HMG-CoA reductase inhibitory activity of the parent compound. Pravastatin undergoes extensive first-pass extraction in the liver (extraction ratio 0.66). Excretion: Approximately 20% of a radiolabeled oral dose is excreted in urine and 70% in the feces. After intravenous administration of radiolabeled pravastatin to normal volunteers, approximately 47% of total body clearance was via renal excretion and 53% by non-renal routes (i.e., biliary excretion and biotransformation). Following single dose oral administration of 14C-pravastatin, the radioactive elimination t½ for pravastatin is 1.8 hours in humans.
Specific Populations
Renal Impairment: A single 20 mg oral dose of pravastatin was administered to 24 patients with varying degrees of renal impairment (as determined by creatinine clearance). No effect was observed on the pharmacokinetics of pravastatin or its 3α-hydroxy isomeric metabolite (SQ 31,906). Compared to healthy subjects with normal renal function, patients with severe renal impairment had 69% and 37% higher mean AUC and Cmax values, respectively, and a 0.61 hour shorter t½ for the inactive enzymatic ring hydroxylation metabolite (SQ 31,945). Hepatic Impairment: In a study comparing the kinetics of pravastatin in patients with biopsy confirmed cirrhosis (N=7) and normal subjects (N=7), the mean AUC varied 18-fold in cirrhotic patients and 5-fold in healthy subjects. Similarly, the peak pravastatin values varied 47-fold for cirrhotic patients compared to 6-fold for healthy subjects. [See Warnings and Precautions (5.2).] Geriatric: In a single oral dose study using pravastatin 20 mg, the mean AUC for pravastatin was approximately 27% greater and the mean cumulative urinary excretion (CUE) approximately 19% lower in elderly men (65 to 75 years old) compared with younger men (19 to 31 years old). In a similar study conducted in women, the mean AUC for pravastatin was approximately 46% higher and the mean CUE approximately 18% lower in elderly women (65 to 78 years old) compared with younger women (18 to 38 years old). In both studies, Cmax, Tmax, and t½ values were similar in older and younger subjects. [See Use in Specific Populations (8.5).] Pediatric: After 2 weeks of once-daily 20 mg oral pravastatin administration, the geometric means of AUC were 80.7 (CV 44%) and 44.8 (CV 89%) ng*hr/mL for children (8 to 11 years, N=14) and adolescents (12 to 16 years, N=10), respectively. The corresponding values for Cmax were 42.4 (CV 54%) and 18.6 ng/mL (CV 100%) for children and adolescents, respectively. No conclusion can be made based on these findings due to the small number of samples and large variability. [See Use in Specific Populations (8.4).]
Drug-Drug Interactions


Pharmacokinetics
There is limited information regarding Pharmacokinetics of Pravastatin in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year study in rats fed pravastatin at doses of 10, 30, or 100 mg/kg body weight, there was an increased incidence of hepatocellular carcinomas in males at the highest dose (p<0.01). These effects in rats were observed at approximately 12 times the human dose (HD) of 80 mg based on body surface area (mg/m2) and at approximately 4 times the HD, based on AUC. In a 2-year study in mice fed pravastatin at doses of 250 and 500 mg/kg/day, there was an increased incidence of hepatocellular carcinomas in males and females at both 250 and 500 mg/kg/day (p<0.0001). At these doses, lung adenomas in females were increased (p=0.013). These effects in mice were observed at approximately 15 times (250 mg/kg/day) and 23 times (500 mg/kg/day) the HD of 80 mg, based on AUC. In another 2-year study in mice with doses up to 100 mg/kg/day (producing drug exposures approximately 2 times the HD of 80 mg, based on AUC), there were no drug-induced tumors. No evidence of mutagenicity was observed in vitro, with or without rat-liver metabolic activation, in the following studies: microbial mutagen tests, using mutant strains of Salmonella typhimurium or Escherichia coli; a forward mutation assay in L5178Y TK +/− mouse lymphoma cells; a chromosomal aberration test in hamster cells; and a gene conversion assay using Saccharomyces cerevisiae. In addition, there was no evidence of mutagenicity in either a dominant lethal test in mice or a micronucleus test in mice. In a fertility study in adult rats with daily doses up to 500 mg/kg, pravastatin did not produce any adverse effects on fertility or general reproductive performance.
Animal Toxicology and/or Pharmacology
CNS Toxicity
CNS vascular lesions, characterized by perivascular hemorrhage and edema and mononuclear cell infiltration of perivascular spaces, were seen in dogs treated with pravastatin at a dose of 25 mg/kg/day. These effects in dogs were observed at approximately 59 times the HD of 80 mg/day, based on AUC. Similar CNS vascular lesions have been observed with several other drugs in this class. A chemically similar drug in this class produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). This same drug also produced vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis in dogs treated for 14 weeks at 180 mg/kg/day, a dose which resulted in a mean plasma drug level similar to that seen with the 60 mg/kg/day dose. When administered to juvenile rats (postnatal days [PND] 4 through 80 at 5 to 45 mg/kg/day), no drug related changes were observed at 5 mg/kg/day. At 15 and 45 mg/kg/day, altered body-weight gain was observed during the dosing and 52-day recovery periods as well as slight thinning of the corpus callosum at the end of the recovery period. This finding was not evident in rats examined at the completion of the dosing period and was not associated with any inflammatory or degenerative changes in the brain. The biological relevance of the corpus callosum finding is uncertain due to the absence of any other microscopic changes in the brain or peripheral nervous tissue and because it occurred at the end of the recovery period. Neurobehavioral changes (enhanced acoustic startle responses and increased errors in water-maze learning) combined with evidence of generalized toxicity were noted at 45 mg/kg/day during the later part of the recovery period. Serum pravastatin levels at 15 mg/kg/day are approximately ≥1 times (AUC) the maximum pediatric dose of 40 mg. No thinning of the corpus callosum was observed in rats dosed with pravastatin (≥250 mg/kg/day) beginning PND 35 for 3 months suggesting increased sensitivity in younger rats. PND 35 in a rat is approximately equivalent to an 8- to 12-year-old human child. Juvenile male rats given 90 times (AUC) the 40 mg dose had decreased fertility (20%) with sperm abnormalities compared to controls.
Clinical Studies
There is limited information regarding Clinical Studies of Pravastatin in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Pravastatin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pravastatin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pravastatin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Pravastatin in the drug label.
Precautions with Alcohol
- Alcohol-Pravastatin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 Neuvonen, PJ; Backman, JT; Niemi, M (2008). "Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin". Clinical Pharmacokinetics. 47 (7): 463–74. doi:10.2165/00003088-200847070-00003. PMID 18563955.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Pravastatin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Pravastatin |Label Name=Pravastatin11.png
}}
{{#subobject:
|Label Page=Pravastatin |Label Name=Pravastatin11.png
}}