Phenoxybenzamine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 98: | Line 98: | ||
|warnings= | |warnings= | ||
* | * Dibenzyline-induced alpha-adrenergic blockade leaves beta-adrenergic receptors unopposed. Compounds that stimulate both types of receptors may, therefore, produce an exaggerated hypotensive response and tachycardia. | ||
====Precautions==== | ====Precautions==== | ||
* | * Administer with caution in patients with marked cerebral or coronary arteriosclerosis or renal damage. Adrenergic blocking effect may aggravate symptoms of respiratory infections. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 111: | Line 111: | ||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 180: | Line 116: | ||
|postmarketing= | |postmarketing= | ||
* The following adverse reactions have been observed, but there are insufficient data to support an estimate of their frequency. | |||
=====Autonomic Nervous System===== | |||
Postural hypotension, tachycardia, inhibition of ejaculation, nasal congestion, miosis. | |||
=====Miscellaneous===== | =====Miscellaneous===== | ||
Gastrointestinal irritation, drowsiness, fatigue. | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
| Line 238: | Line 130: | ||
|drugInteractions= | |drugInteractions= | ||
* | * Dibenzyline (phenoxybenzamine hydrochloride) may interact with compounds that stimulate both alpha- and beta-adrenergic receptors (i.e., epinephrine) to produce an exaggerated hypotensive response and tachycardia. | ||
* Dibenzyline blocks hyperthermia production by levarterenol, and blocks hypothermia production by reserpine. | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category C''' | ||
:* Adequate reproductive studies in animals have not been performed with Dibenzyline (phenoxybenzamine hydrochloride). It is also not known whether Dibenzyline can cause fetal harm when administered to a pregnant woman. Dibenzyline should be given to a pregnant woman only if clearly needed. | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 255: | Line 148: | ||
|useInNursing= | |useInNursing= | ||
* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions from phenoxybenzamine hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed= | |useInPed= | ||
* Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri= | |useInGeri= | ||
| Line 408: | Line 301: | ||
|nonClinToxic= | |nonClinToxic= | ||
* Case reports of carcinoma in humans after long-term treatment with phenoxybenzamine have been reported. Hence long-term use of phenoxybenzamine is not recommended. Carefully weigh the benefits and risks before prescribing this drug. | |||
* Phenoxybenzamine hydrochloride showed in vitro mutagenic activity in the Ames test and mouse lymphoma assay; it did not show mutagenic activity in vivo in the micronucleus test in mice. In rats and mice, repeated intraperitoneal administration of phenoxybenzamine hydrochloride (three times per week for up to 52 weeks) resulted in peritoneal sarcomas. Chronic oral dosing in rats (for up to 2 years) produced malignant tumors of the small intestine and non-glandular stomach, as well as ulcerative and/or erosive gastritis of the glandular stomach. Whereas squamous cell carcinomas of the non-glandular stomach were observed at all tested doses of phenoxybenzamine hydrochloride, there was a no-observed-effect-level of 10 mg/kg for tumors (carcinomas and sarcomas) of the small intestine. This dose is, on a body surface area basis, about twice the maximum recommended human dosage of 20 mg b.i.d. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
Revision as of 16:04, 26 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Phenoxybenzamine is a vasodilator and alpha-adrenergic blocker that is FDA approved for the {{{indicationType}}} of pheochromocytoma, to control episodes of hypertension and sweating.. Common adverse reactions include hypotension, tachyarrhythmia, nausea, vomiting, xerostomia, dizziness, somnolence, miosis, nasal congestion, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Pheochromocytoma
- Dosing Information
- * The dosage should be adjusted to fit the needs of each patient. Small initial doses should be slowly increased until the desired effect is obtained or the side effects from blockade become troublesome. After each increase, the patient should be observed on that level before instituting another increase. The dosage should be carried to a point where symptomatic relief and/or objective improvement are obtained, but not so high that the side effects from blockade become troublesome.
- Initially, 10 mg of Dibenzyline (phenoxybenzamine hydrochloride) twice a day. Dosage should be increased every other day, usually to 20 to 40 mg 2 or 3 times a day, until an optimal dosage is obtained, as judged by blood pressure control.
- * Long-term use of phenoxybenzamine is not recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenoxybenzamine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenoxybenzamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenoxybenzamine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenoxybenzamine in pediatric patients.
Contraindications
- Conditions where a fall in blood pressure may be undesirable
- Hypersensitivity to the drug or any of its components.
Warnings
- Dibenzyline-induced alpha-adrenergic blockade leaves beta-adrenergic receptors unopposed. Compounds that stimulate both types of receptors may, therefore, produce an exaggerated hypotensive response and tachycardia.
Precautions
- Administer with caution in patients with marked cerebral or coronary arteriosclerosis or renal damage. Adrenergic blocking effect may aggravate symptoms of respiratory infections.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Phenoxybenzamine in the drug label.
Postmarketing Experience
- The following adverse reactions have been observed, but there are insufficient data to support an estimate of their frequency.
Autonomic Nervous System
Postural hypotension, tachycardia, inhibition of ejaculation, nasal congestion, miosis.
Miscellaneous
Gastrointestinal irritation, drowsiness, fatigue.
Drug Interactions
- Dibenzyline (phenoxybenzamine hydrochloride) may interact with compounds that stimulate both alpha- and beta-adrenergic receptors (i.e., epinephrine) to produce an exaggerated hypotensive response and tachycardia.
- Dibenzyline blocks hyperthermia production by levarterenol, and blocks hypothermia production by reserpine.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Adequate reproductive studies in animals have not been performed with Dibenzyline (phenoxybenzamine hydrochloride). It is also not known whether Dibenzyline can cause fetal harm when administered to a pregnant woman. Dibenzyline should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Phenoxybenzamine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Phenoxybenzamine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions from phenoxybenzamine hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Phenoxybenzamine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Phenoxybenzamine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Phenoxybenzamine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Phenoxybenzamine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Phenoxybenzamine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Phenoxybenzamine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Phenoxybenzamine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Phenoxybenzamine in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Phenoxybenzamine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Phenoxybenzamine in the drug label.
Pharmacology
| |
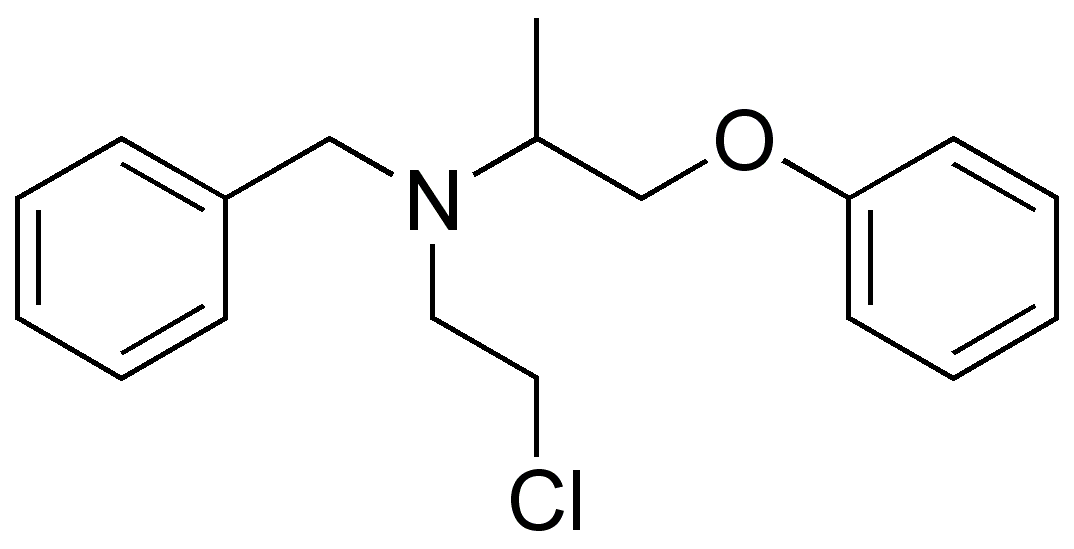
1 : 1 mixture (racemate)Phenoxybenzamine
| |
| Systematic (IUPAC) name | |
| (RS)-N-benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine | |
| Identifiers | |
| CAS number | |
| ATC code | C04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 303.826 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 24 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C (U.S.) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Phenoxybenzamine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Phenoxybenzamine in the drug label.
Nonclinical Toxicology
- Case reports of carcinoma in humans after long-term treatment with phenoxybenzamine have been reported. Hence long-term use of phenoxybenzamine is not recommended. Carefully weigh the benefits and risks before prescribing this drug.
- Phenoxybenzamine hydrochloride showed in vitro mutagenic activity in the Ames test and mouse lymphoma assay; it did not show mutagenic activity in vivo in the micronucleus test in mice. In rats and mice, repeated intraperitoneal administration of phenoxybenzamine hydrochloride (three times per week for up to 52 weeks) resulted in peritoneal sarcomas. Chronic oral dosing in rats (for up to 2 years) produced malignant tumors of the small intestine and non-glandular stomach, as well as ulcerative and/or erosive gastritis of the glandular stomach. Whereas squamous cell carcinomas of the non-glandular stomach were observed at all tested doses of phenoxybenzamine hydrochloride, there was a no-observed-effect-level of 10 mg/kg for tumors (carcinomas and sarcomas) of the small intestine. This dose is, on a body surface area basis, about twice the maximum recommended human dosage of 20 mg b.i.d.
Clinical Studies
There is limited information regarding Clinical Studies of Phenoxybenzamine in the drug label.
How Supplied
- Dibenzyline (phenoxybenzamine hydrochloride) capsules, 10 mg, in bottles of 100 (NDC 65197-001-01).
- Store at 25°C (77°F); excursions permitted to 15°- 30°C (59°- 86°F).
Storage
There is limited information regarding Phenoxybenzamine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Phenoxybenzamine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Phenoxybenzamine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Phenoxybenzamine in the drug label.
Precautions with Alcohol
- Alcohol-Phenoxybenzamine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dibenzyline®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "DIBENZYLINE (phenoxybenzamine hydrochloride) capsule".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Phenoxybenzamine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Phenoxybenzamine |Label Name=Phenoxybenzamine11.png
}}
{{#subobject:
|Label Page=Phenoxybenzamine |Label Name=Phenoxybenzamine11.png
}}