Pentagastrin: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Pentagastrin": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag=<!--Overview-->{{RB}} | |authorTag=<!--Overview-->{{RB}} | ||
|genericName=Pentagastrin | |genericName=Pentagastrin | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=diagnostic aid | |drugClass=diagnostic aid | ||
|indicationType=diagnosis | |indicationType=diagnosis | ||
|indication=Anacidity, Hypersecretory conditions | |indication=[[Anacidity]], [[Hypersecretory conditions]] | ||
|adverseReactions=Nausea, abdominal cramps | |adverseReactions=[[Nausea]], [[abdominal cramps]] | ||
| Line 19: | Line 19: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=====Indications==== | |fdaLIADAdult=====Indications==== | ||
Anacidity (diagnosis)—Pentagastrin is indicated as a diagnostic aid for evaluation of gastric acid secretory function. It is effective in testing for anacidity (achlorhydria) in patients with suspected pernicious anemia, atrophic gastritis, or gastric carcinoma. It is also effective in determining the reduction in acid output after operations for peptic ulcer, such as vagotomy or gastric resection. | * [[Anacidity]] (diagnosis)—Pentagastrin is indicated as a diagnostic aid for evaluation of [[gastric acid]] secretory function. It is effective in testing for anacidity ([[achlorhydria]]) in patients with suspected [[pernicious anemia]], [[atrophic gastritis]], or [[gastric carcinoma]]. It is also effective in determining the reduction in acid output after operations for peptic ulcer, such as vagotomy or gastric resection. | ||
Hypersecretory conditions, gastric (diagnosis)—Pentagastrin is indicated as a diagnostic aid in testing for gastric hypersecretion in patients with suspected duodenal ulcer or postoperative stomal ulcer, and for the diagnosis of Zollinger-Ellison tumor | * [[Hypersecretory conditions]], gastric (diagnosis)—Pentagastrin is indicated as a diagnostic aid in testing for gastric hypersecretion in patients with suspected [[duodenal ulcer]] or postoperative [[stomal ulcer]], and for the diagnosis of [[Zollinger-Ellison tumor]] | ||
====Dosage==== | ====Dosage==== | ||
* The intravenous infusion dose has ranged from 0.1 to 12 mcg (0.0001 to 0.012 mg) per kg of body weight per hour administered in a 0.9% sodium chloride injection. It can also be used as a subcutaneous injection for gastric function study with a dose of 6 mcg (0.006 mg) per kg of body weight. | * The [[intravenous]] infusion dose has ranged from 0.1 to 12 mcg (0.0001 to 0.012 mg) per kg of body weight per hour administered in a 0.9% sodium chloride injection. It can also be used as a subcutaneous injection for gastric function study with a dose of 6 mcg (0.006 mg) per kg of body weight. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 43: | Line 43: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications=<!--Warnings--> | ||
|warnings=<!--Adverse Reactions--> | |||
<!--Warnings--> | |||
|warnings= | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=* Nausea<ref name="pmid5481572">{{cite journal| author=Barrowman JA, Herxheimer A, Kits TP| title=Unwanted effects of pentagastrin. | journal=Clin Pharmacol Ther | year= 1970 | volume= 11 | issue= 6 | pages= 862-8 | pmid=5481572 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5481572 }} </ref> | |clinicalTrials=* [[Nausea]]<ref name="pmid5481572">{{cite journal| author=Barrowman JA, Herxheimer A, Kits TP| title=Unwanted effects of pentagastrin. | journal=Clin Pharmacol Ther | year= 1970 | volume= 11 | issue= 6 | pages= 862-8 | pmid=5481572 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5481572 }} </ref> | ||
* Abdominal cramps | * [[Abdominal cramps]] | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
| Line 110: | Line 56: | ||
|drugInteractions=* The following may affect pentagastrin’s action: | |drugInteractions=* The following may affect pentagastrin’s action: | ||
Antacids, anticholinergics, histamine H2-receptor antagosnists, or omeprazole | * [[Antacids]], [[anticholinergics]], histamine [[H2-receptor antagosnists]], or [[omeprazole]] | ||
Acute, obstructing, penetrating or bleeding peptic ulcers | * Acute, obstructing, penetrating or bleeding [[peptic ulcers]] | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
| Line 130: | Line 76: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* intravenous infusion | |administration=* intravenous infusion | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 144: | Line 90: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox=<!--Mechanism of Action--> | |drugBox={{Drugbox2 | ||
| Watchedfields = changed | |||
| verifiedrevid = 464198043 | |||
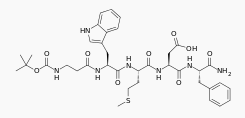
| IUPAC_name = ''N''-(tert-butoxycarbonyl)-β-alanyl-<small>L</small>-tryptophyl-<small>L</small>-methionyl-<small>L</small>-α-aspartyl-<small>L</small>-phenylalaninamide<ref>{{cite book|last1=Martindale|title=The extra pharmacopoeia|date=1993|publisher=Pharmaceutical Press|location=London|isbn=978-0853693000|edition=30th}}</ref> | |||
| image = Pentagastrin Wiki Str.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|CONS|pentagastrin}} | |||
| pregnancy_category = | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = 10 minutes or less | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 5534-95-2 | |||
| ATC_prefix = V04 | |||
| ATC_suffix = CG04 | |||
| ATC_supplemental = | |||
| PubChem = 9853654 | |||
| IUPHAR_ligand = 870 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00183 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 8029364 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = EF0NX91490 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D01631 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1328 | |||
<!--Chemical data--> | |||
| C=37 | H=49 | N=7 | O=9 | S=1 | |||
| molecular_weight = 767.893 g/mol | |||
| smiles = O=C(N)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCNC(=O)OC(C)(C)C)Cc2c1ccccc1nc2)CCSC)CC(=O)O)Cc3ccccc3 | |||
| InChI = 1/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | |||
| InChIKey = NEYNJQRKHLUJRU-DZUOILHNBP | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = NEYNJQRKHLUJRU-DZUOILHNSA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction=* The exact mechanism by which Pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since Pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion. | |mechAction=* The exact mechanism by which Pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since Pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion. | ||
| Line 150: | Line 148: | ||
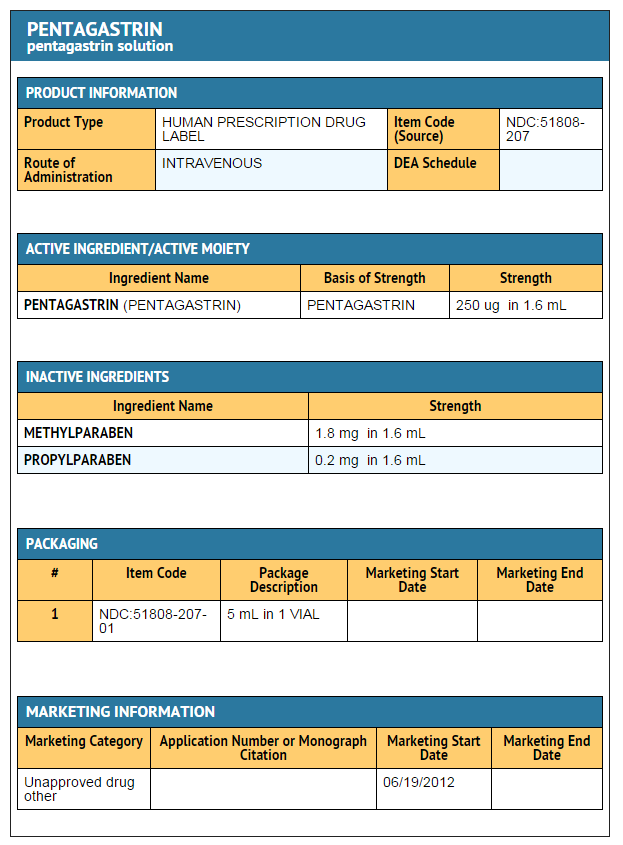
|structure=* Pentagastrin, a diagnostic aid, is supplied as a sterile solution (1.1 ml/5 ml vial) containing: | |structure=* Pentagastrin, a diagnostic aid, is supplied as a sterile solution (1.1 ml/5 ml vial) containing: | ||
250 micrograms Pentagastrin per ml | : 250 micrograms Pentagastrin per ml | ||
0.8 mg Methylparaben and 0.2 mg Propylparaben per mL | : 0.8 mg Methylparaben and 0.2 mg Propylparaben per mL | ||
0.9 mg sodium chloride per mL | : 0.9 mg sodium chloride per mL | ||
pH 8 | : pH 8 | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 161: | Line 158: | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=The exact mechanism by which Pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since Pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion. | |PK=* The exact mechanism by which Pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since Pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion. | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
| Line 170: | Line 167: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied= | |howSupplied= | ||
|storage=* Keep refrigerated between 2◦ and 8◦C. Protect from light | |storage=* Keep refrigerated between 2◦ and 8◦C. Protect from light | ||
|packLabel=====PACKAGE LABEL.PRINCIPAL DISPLAY PANEL==== | |packLabel=====PACKAGE LABEL.PRINCIPAL DISPLAY PANEL==== | ||
| Line 191: | Line 188: | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 16:54, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pentagastrin is a diagnostic aid that is FDA approved for the diagnosis of Anacidity, Hypersecretory conditions. Common adverse reactions include Nausea, abdominal cramps.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Anacidity (diagnosis)—Pentagastrin is indicated as a diagnostic aid for evaluation of gastric acid secretory function. It is effective in testing for anacidity (achlorhydria) in patients with suspected pernicious anemia, atrophic gastritis, or gastric carcinoma. It is also effective in determining the reduction in acid output after operations for peptic ulcer, such as vagotomy or gastric resection.
- Hypersecretory conditions, gastric (diagnosis)—Pentagastrin is indicated as a diagnostic aid in testing for gastric hypersecretion in patients with suspected duodenal ulcer or postoperative stomal ulcer, and for the diagnosis of Zollinger-Ellison tumor
Dosage
- The intravenous infusion dose has ranged from 0.1 to 12 mcg (0.0001 to 0.012 mg) per kg of body weight per hour administered in a 0.9% sodium chloride injection. It can also be used as a subcutaneous injection for gastric function study with a dose of 6 mcg (0.006 mg) per kg of body weight.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pentagastrin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pentagastrin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Pentagastrin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pentagastrin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pentagastrin in pediatric patients.
Contraindications
There is limited information regarding Pentagastrin Contraindications in the drug label.
Warnings
There is limited information regarding Pentagastrin Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Pentagastrin in the drug label.
Drug Interactions
- The following may affect pentagastrin’s action:
- Antacids, anticholinergics, histamine H2-receptor antagosnists, or omeprazole
- Acute, obstructing, penetrating or bleeding peptic ulcers
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pentagastrin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pentagastrin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pentagastrin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Pentagastrin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Pentagastrin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Pentagastrin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pentagastrin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pentagastrin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pentagastrin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pentagastrin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pentagastrin in patients who are immunocompromised.
Administration and Monitoring
Administration
- intravenous infusion
Monitoring
There is limited information regarding Monitoring of Pentagastrin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Pentagastrin in the drug label.
Overdosage
There is limited information regarding Overdose of Pentagastrin in the drug label.
Pharmacology
| |
Pentagastrin
| |
| Systematic (IUPAC) name | |
| N-(tert-butoxycarbonyl)-β-alanyl-L-tryptophyl-L-methionyl-L-α-aspartyl-L-phenylalaninamide[2] | |
| Identifiers | |
| CAS number | |
| ATC code | V04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 767.893 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 10 minutes or less |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- The exact mechanism by which Pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since Pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion.
Structure
- Pentagastrin, a diagnostic aid, is supplied as a sterile solution (1.1 ml/5 ml vial) containing:
- 250 micrograms Pentagastrin per ml
- 0.8 mg Methylparaben and 0.2 mg Propylparaben per mL
- 0.9 mg sodium chloride per mL
- pH 8
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Pentagastrin in the drug label.
Pharmacokinetics
- The exact mechanism by which Pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since Pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Pentagastrin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Pentagastrin in the drug label.
How Supplied
There is limited information regarding Pentagastrin How Supplied in the drug label.
Storage
- Keep refrigerated between 2◦ and 8◦C. Protect from light
Images
Drug Images
{{#ask: Page Name::Pentagastrin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Pentagastrin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Pentagastrin in the drug label.
Precautions with Alcohol
- Alcohol-Pentagastrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PENTAGASTRIN ®[3]
Look-Alike Drug Names
There is limited information regarding Pentagastrin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Barrowman JA, Herxheimer A, Kits TP (1970). "Unwanted effects of pentagastrin". Clin Pharmacol Ther. 11 (6): 862–8. PMID 5481572.
- ↑ Martindale (1993). The extra pharmacopoeia (30th ed.). London: Pharmaceutical Press. ISBN 978-0853693000.
- ↑ "PENTAGASTRIN".