Penicillin G potassium
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
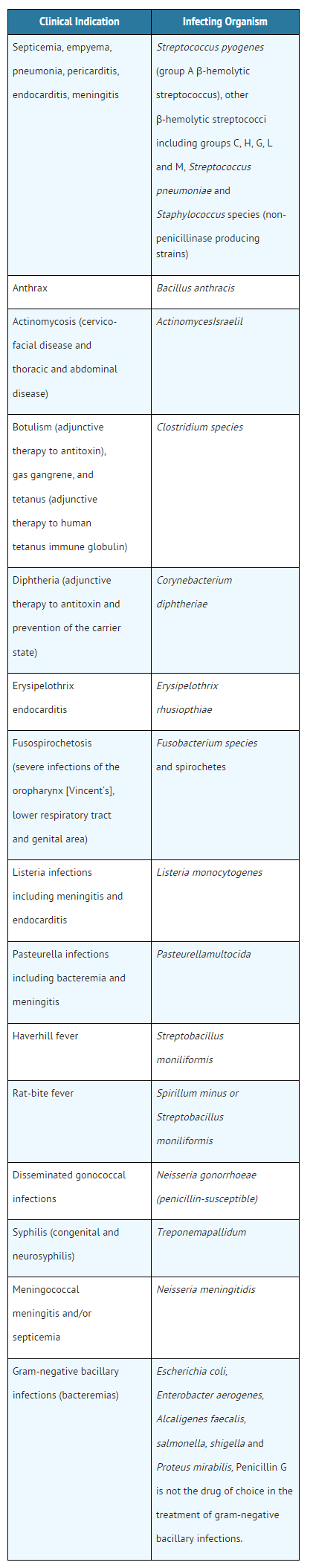
Penicillin G potassium is an antibiotic that is FDA approved for the treatment of serious infections caused by susceptible strains of the designated microorganisms causing septicemia, empyema, pneumonia, pericarditis, endocarditis, meningitis, anthrax, actinomycosis, botulism, diphtheria, erysipelothrix, endocarditis, fusospirochetosis. Common adverse reactions include hypersensitivity reactions, hemolytic anemia, nausea, vomiting, stomatitis, neutropenia, hypereflexia, seizures, interstitial nephritis, thrombophlebitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Therapy
- Penicillin G Sodium for Injection, USP is indicated in the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
- Appropriate culture and susceptibility tests should be done before treatment in order to isolate and identify organisms causing infection and to determine their susceptibility to penicillin G. Therapy with Penicillin G Sodium for Injection, USP may be initiated before results of such tests are known when there is reason to believe the infection may involve any of the organisms listed below, however, once these results become available, appropriate therapy should be continued.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of penicillin G sodium and other antibacterial drugs, penicillin G sodium should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
- When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
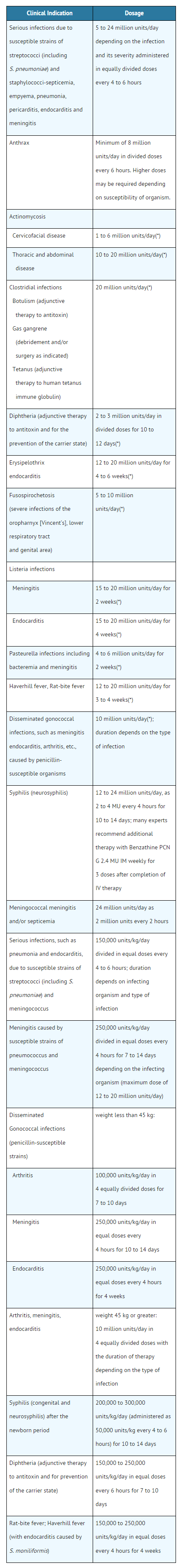
- Penicillin G Sodium for lnjection, USP may be given intravenously or intramuscularly. The usual dose recommendations are as follows:
- Because of its short half-life, Penicillin G is administered in divided doses, usually every 4 to 6 hours with the exception of meningococcal meningitis/septicemia, i.e., every 2 hours.
The recommended dosage regimens are as follows
- Creatinine clearance less than 10 mL/min/1.73 m2; administer a full loading dose followed by one-half of the loading dose every 8 to 10 hours.
- Uremic patients with a creatinine clearance greater than 10 mL/min/1.73m2; administer a full loading dose followed by one-half of the loading dose every 4 to 5 hours.
- Additional dosage modifications should be made in patients with hepatic disease and renal impairment.
- For most acute infections, treatment should be continued for at least 48 to 72 hours after the patient becomes asymptomatic. Antibiotic therapy for Group A β-hemolytic streptococcal infections should be maintained for at least 10 days to reduce the risk of rheumatic fever.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
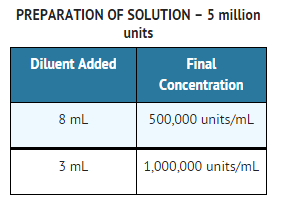
Preparation of Solution
- Solutions of penicillin should be prepared as follows
- Loosen powder. Hold vial horizontally and rotate it while slowly directing the stream of diluent against the wall of the vial. Shake vial vigorously after all the diluent has been added. Depending on the route of administration, use Sterile Water for Injection, USP, 0.9% Sodium Chloride Injection, USP, or Dextrose Injections, USP.
- Penicillins are rapidly inactivated in the presence of carbohydrate solutions at alkaline pH.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Lyme disease[1]
- Streptococcus group B infection of the infant, Intrapartum; Prophylaxis[2]
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penicillin G potassium in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- This product should not be administered to patients requiring less than one million units per dose.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Streptococcus group B infection of the infant, Intrapartum; Prophylaxis[2]
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penicillin G potassium in pediatric patients.
Contraindications
- A history of hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
Warnings
There is limited information regarding Penicillin G potassium Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Penicillin G potassium in the drug label.
Postmarketing Experience
Body as a Whole
- The Jarisch-Herxheimer reaction is a systemic reaction, that may occur after the initiation of penicillin therapy in patients with syphilis or other spirochetal infections (i.e., Lyme disease and Relapsing fever). The reaction begins one to two hours after initiation of therapy and disappears within 12 to 24 hours. It is characterized by fever, chills, myalgias, headache, exacerbation of cutaneous lesions, tachycardia, hyperventiliation, vasodilation with flushing and mild hypotension. The pathogenesis of the Herxheimer reaction may be due to the release from the spirochaete of host stable pyrogen.
Hypersensitivity Reactions
- The reported incidence of allergic reactions to all penicillins ranges from 0.7 to 10 percent in different studies. Sensitization is usually the result of previous treatment with a penicillin, but some individuals have had immediate reactions when first treated. In such cases, it is postulated that prior exposure to penicillin may have occurred via trace amounts present in milk or vaccines.
- Two types of allergic reactions to penicillin are noted clinically – Immediate and delayed.
- Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angloneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death.
- Such immediate anaphylactic reactions are very rare and usually occur after parenteral therapy, but a few cases of anaphylaxis have been reported following oral therapy. Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 45 hours after administration and may include urticaria, pruritus, fever and, occasionally, laryngeal edema.
- Delayed reactions to penicillin therapy usually occur within 1 to 2 weeks after initiation of therapy. Manifestations include serum sickness-like symptoms, i.e., fever, malaise, urticaria, myalgia, arthralgia, abdominal pain and various skin rashes, ranging from maculopapular eruptions to exfoliative dermatitis.
- Contact dermatitis has been observed in individuals who prepare penicillin solutions.
Gastrointestinal System
- Pseudomembranous colitis has been reported with the onset occurring during or after penicillin G treatment. Nausea, vomiting, stomatitis, black or hairy tongue, and other symptoms of gastrointestinal irritation may occur, especially during oral therapy.
Hematologic System
- Reactions include neutropenia, which resolves after penicillin therapy is discontinued; Coombs-positive hemolytic anemia, an uncommon reaction, occurs in patients treated with intravenous penicillin G in doses greater than 10 million units/day and who have previously received large doses of the drug; and with large doses of penicillin, a bleeding diathesis, can occur secondary to platelet dysfunction.
Metabolic
- Penicillin G Sodium for lnjection, USP (1 million units contains 1.68 mEq of sodium ion) may cause serious and even fatal electrolyte disturbances when given intravenously in large doses.
Cardiovascular System
- High dosage of penicillin G sodium may result in congestive heart failure due to high sodium intake.
Nervous System
- Neurotoxic reactions including hyperreflexia, myoclonic twitches, seizures and coma have been reported following the administration of massive intravenous doses, and are more likely in patients with impaired renal function.
Urogenital System
- Renal tubular damage and interstitial nephritis have been associated with large intravenous doses of penicillin G. Manifestations of this reaction may include fever, rash, eosinophilia, proteinuria, eosinophiluria, hematuria and a rise in serum urea nitrogen. Discontinuation of penicillin G results in resolution in the majority of patients.
Local Reactions
- Phlebitis and thrombophlebitis may occur with intravenous administration.
Drug Interactions
There is limited information regarding Penicillin G potassium Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Penicillin G potassium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Penicillin G potassium during labor and delivery.
Nursing Mothers
- Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
- lncompletely developed renal function in newborns may delay elimination of penicillin; therefore, appropriate reductions in the dosage and frequency of administration should be made in these patients. All newborns treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects.
- Pediatric doses are generally determined on a weight basis and should be calculated for each patient individually.
- The potential for toxic effects in children from chemicals that may leach from the single dose premixed intravenous preparation in plastic containers has not been evaluated.
Geriatic Use
There is no FDA guidance on the use of Penicillin G potassium with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Penicillin G potassium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Penicillin G potassium with respect to specific racial populations.
Renal Impairment
- Penicillin G is relatively nontoxic, and dosage adjustments are generally required only in cases of severe renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Penicillin G potassium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Penicillin G potassium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Penicillin G potassium in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- All newborns treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects.
IV Compatibility
There is limited information regarding IV Compatibility of Penicillin G potassium in the drug label.
Overdosage
- Dose related toxicity may arise with the use of massive doses of intravenous penicillins (40 to 100 million units per day), particularly in patients with severe renal impairment.
- The manifestations may include agitation, confusion, asterixis, hallucinations, stupor, coma, multifocal myocionus, seizures and encephalopathy. Hyperkalemia is also possible.
- In case of overdosage, discontinue penicillin, treat symptomatically and institute supportive measures as required. If necessary, hemodialysis may be used to reduce blood levels of penicillin G, although the degree of effectiveness of this procedure is questionable.
Pharmacology
Mechanism of Action
There is limited information regarding Penicillin G potassium Mechanism of Action in the drug label.
Structure
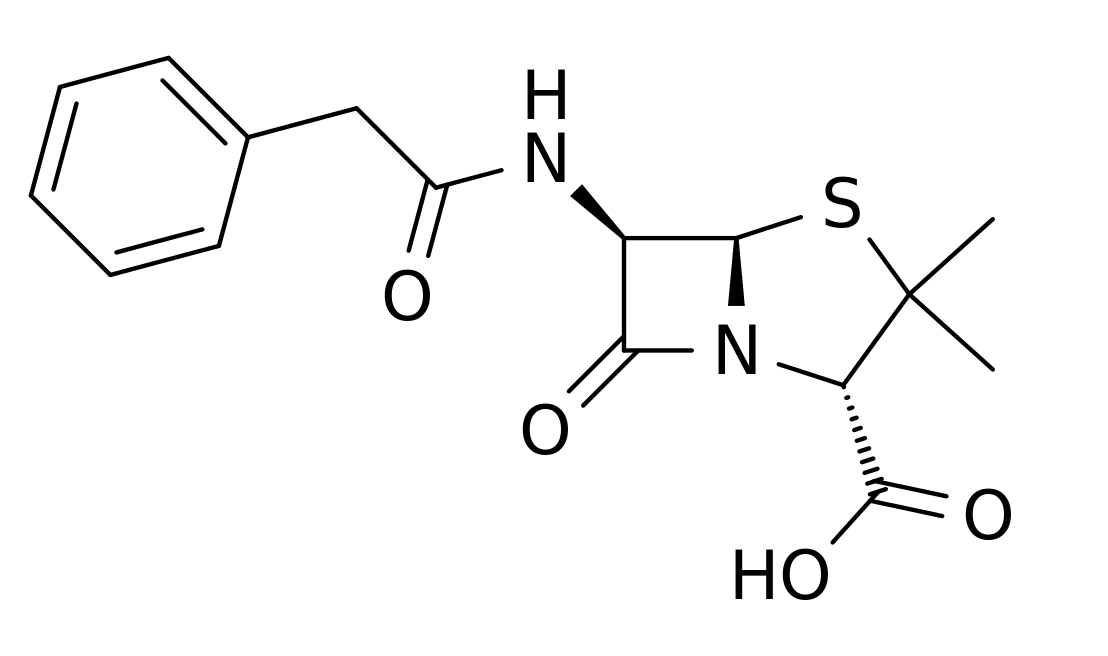
- Penicillin G sodium for Injection, USP is sterile penicillin G sodium powder for reconstitution. It is an antibacterial agent intended for intravenous or intramuscularly use.
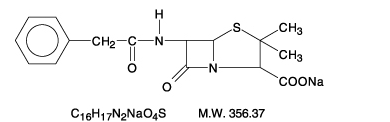
- Chemically, penicillin G sodium is designated 4-Thia-1-azabicyclo(3.2.0)-heptane-2-carboxylic acid,3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]-, [2S -(2α, 5α, 6β)]-, monosodium salt and has the following structural formula:
- Penicillin G sodium, a water soluble benzylpenicillin, is a white to almost white crystalline powder which is almost odorless and/or after reconstitution a colorless solution. The pH of freshly constituted solutions usually ranges from 5.0 to 7.5.
- Penicillin G sodium for Injection, USP is supplied in vials equivalent to 5,000,000 units (5 million units) of penicillin G as the sodium salt, with 1.68 mEq of sodium per million units of penicillin G.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Penicillin G potassium in the drug label.
Pharmacokinetics
- After an intravenous infusion of penicillin G, peak serum concentrations are attained immediately after completion of the infusion. In a study of ten patients administered a single 5 million unit dose of penicillin G intravenously over 3 to 5 minutes, the mean serum concentrations were 400 mcg/mL, 273 mcg/mL and 3 mcg/mL at 5 to 6 minutes, 10 minutes and 4 hours after completion of the injection, respectively.
- In a separate study, five healthy adults were administered one million units of penicillin G intravenously, either as a bolus over 4 minutes or as an infusion over 60 minutes. The mean serum concentration eight minutes after completion of the bolus was 45 mcg/mL and eight minutes after completion of the infusion was 14.4 mcg/mL.
- The mean β-phase serum half-life of penicillin G administered by the intravenous route in ten patients with normal renal function was 42 minutes, with a range of 31 to 50 minutes.
- The clearance of penicillin G in normal individuals is predominantly via the kidney. The renal clearance, which is extremely rapid, is the result of glomerular filtration and active tubular transport, with the latter route predominating. Urinary recovery is reported to be 58 to 85% of the administered dose. Renal clearance of penicillin is delayed in premature infants, neonates and in the elderly due to decreased renal function. The serum half-life of penicillin G correlates inversely with age and clearance of creatinine and ranges from 3.2 hours in infants 0 to 6 days of age to 1.4 hours in infants 14 days of age or older.
- Nonrenal clearance includes hepatic metabolism and, to a lesser extent, biliary excretion. The latter routes become more important with renal impairment.
- Probenecid blocks the renal tubular secretion of penicillin. Therefore, the concurrent administration of probenecid prolongs the elimination of penicillin G and, consequently, increases the serum concentrations.
- Penicillin G is distributed to more areas of the body including lung, liver, kidney, muscle, bone and placenta. In the presence of inflammation, levels of penicillin in abscesses, middle ear, pleural, peritoneal and synovial fluids are sufficient to inhibit most susceptible bacteria. Penetration into the eye, brain, cerebrospinal fluid (CSF) or prostate is poor in the absence of inflammation. With inflamed meninges, the penetration of penicillin G into the CSF improves, such that the CSF/serum ratio is 2 to 6%. Inflammation also enhances its penetration into the pericardial fluid. Penicillin G is actively secreted into the bile resulting in levels at least 10 times those achieved simultaneously in serum. Penicillin G penetrates poorly into human polymorphonuclear leukocytes.
- In the presence of impaired renal function, the β-phase serum half-life of penicillin G is prolonged. β-phase serum half-lives of one to two hours were observed in azotemic patients with serum creatinine concentrations <3 mg/100 mL and ranged as high as 20 hours in anuric patients. A linear relationship, including the lowest range of renal function, is found between the serum elimination rate constant and renal function as measured by creatinine clearance.
- In patients with altered renal function, the presence of hepatic insufficiency further alters the elimination of penicillin G. In one study, the serum half-lives in two anuric patients (excreting <400 mL urine/day) were 7.2 and 10.1 hours. A totally anuric patient with terminal hepatic cirrhosis had a penicillin half-life of 30.5 hours, while another patient with anuria and liver disease had a serum half-life of 16.4 hours.The dosage of penicillin G should be reduced in patients with severe renal impairment, with additional modifications when hepatic disease accompanies the renal impairment.
- Hemodialysis has been shown to reduce penicillin G serum levels.
Nonclinical Toxicology
- Penicillin G is bactericidal against penicillin-susceptible microorganisms during the stage of active multiplication. It acts by inhibiting biosynthesis of cell-wall mucopeptide. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci. Penicillin G is highly active in vitro against staphylococci (except penicillinase-producing strains), streptococci (groups A, B, C, G, H, L and M), pneumococci and Neisseria meningitidis. Other organisms susceptible in vitro to penicillin G are Neisseria gonorrhoeae, Corynebacteriumdiphtheriae, Bacillus anthracis, clostridia, Actinomyces species, Spirillum minus, Streptobacillusmonillformis, Listeria monocytogenes, and leptospira; Treponemapallidum is extremely susceptible.
- Some species of gram-negative bacilli were previously considered susceptible to very high intravenous doses of penicillin G (up to 80 million units/day) including some strains of Escherichia coli, Proteus mirabilis, salmonella, shigella, Enterobacteraerogenes (formerly Aerobacteraerogenes) and Alcaligenesfaecalis. Penicillin G is no longer considered a drug of choice for infections caused by these organisms.
Susceptibility Testing
Diffusion Techniques
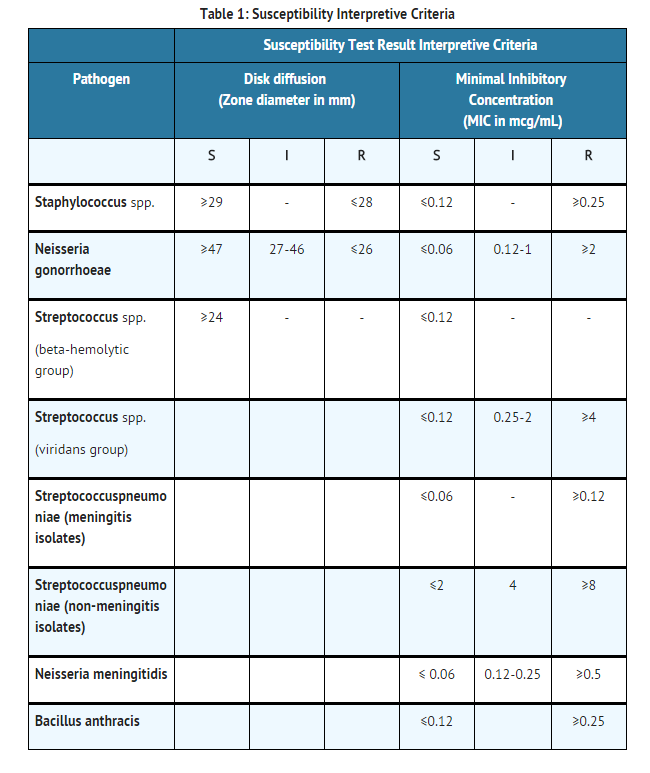
- Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure1,2 which has been recommended for use with disks to test susceptibility of organisms to penicillin G uses the 10 Unit (U) penicillin disk. Interpretation involves the correlation of the diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for penicillin G.
- Reports from the laboratory providing results of the standard single-disk susceptibility test with a 10 U penicillin disk should be interpreted according to the criteria provided in TABLE 1.
Dilution Techniques
- Quantitative methods that are used to determine minimum inhibitory concentrations (MICs) provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure1,3 uses a standardized dilution method (broth or agar) or equivalent with penicillin powder. The MIC values obtained should be interpreted according to the criteria provided in below table.
- A report of “susceptible” indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in the blood. A report of “Intermediate” (I) indicates that the result should be considered equivocal, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of the drug can be used.
- This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
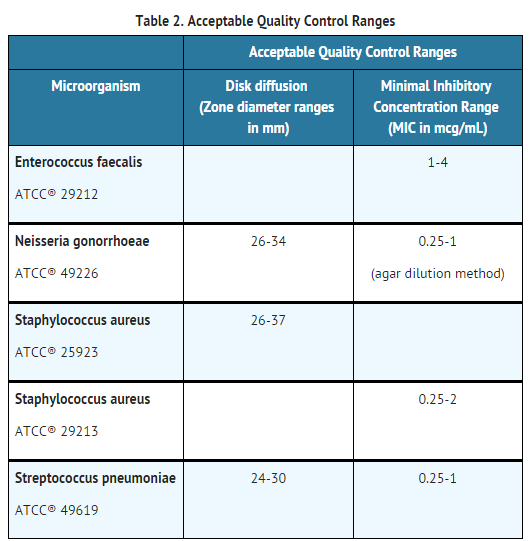
Quality Control
- Standardized susceptibility test procedures require the use of laboratory control microorganisms1,2,3. The 10 U penicillin G disk and the standard penicillin powder should provide respectively the following zone diameters and MIC values in these laboratory test quality control strains:
Clinical Studies
There is limited information regarding Clinical Studies of Penicillin G potassium in the drug label.
How Supplied
- Penicillin G Sodium for lnjection, USP, for IM or IV use, is supplied in dry powder form in vials containing 5,000,000 units (5 million units) of crystalline penicillin G as the sodium salt, with 1.68 mEq of sodium per million units of penicillin G, in tray packs of 10 (NDC 0781-6153-95) and in tray packs of 50 (NDC 0781-6153-97).
Storage
- Store dry powder at 20°-25°C (68°-77°F).
- Sterile constituted solution may be kept in refrigerator (2° to 8°C) for 3 days without significant loss of potency.
Images
Drug Images
{{#ask: Page Name::Penicillin G potassium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

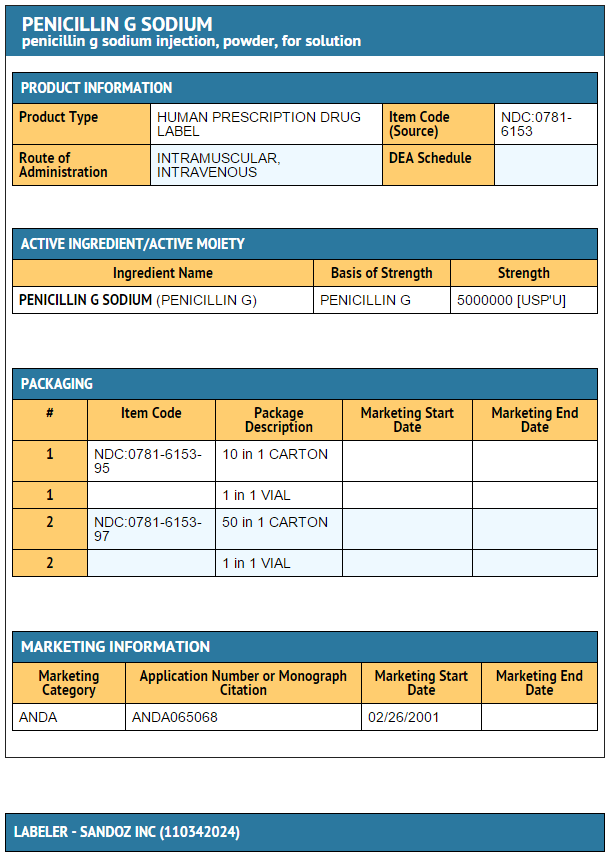
{{#ask: Label Page::Penicillin G potassium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Penicillin G potassium in the drug label.
Precautions with Alcohol
- Alcohol-Penicillin G potassium interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PENICILLIN G SODIUM®[3]
Look-Alike Drug Names
There is limited information regarding Penicillin G potassium Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS; et al. (2006). "The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America". Clin Infect Dis. 43 (9): 1089–134. doi:10.1086/508667. PMID 17029130.
- ↑ 2.0 2.1 "Centers for Disease Control and Prevention".
- ↑ "PENICILLIN G SODIUM- penicillin g sodium injection, powder, for solution".
{{#subobject:
|Label Page=Penicillin G potassium |Label Name=Ampicillin07.jpg
}}
{{#subobject:
|Label Page=Penicillin G potassium |Label Name=DailyMed - PENICILLIN G SODIUM- penicillin g sodium injection, powder, for solution (1).png
}}
_