Penciclovir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
ConditionName:
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Penciclovir is a {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- DENAVIR is a nucleoside analog HSV DNA polymerase inhibitor indicated for the treatment of recurrent herpes labialis (cold sores) in adults and children 12 years of age or older.
Dosage
- DENAVIR should be applied every 2 hours during waking hours for a period of 4 days. Treatment should be started as early as possible (i.e., during the prodrome or when lesions appear).
DOSAGE FORMS & STRENGTHS
- Each gram of DENAVIR contains 10 mg of penciclovir in a cream base, which is equivalent to 1% (w/w).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penciclovir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penciclovir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Penciclovir in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penciclovir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penciclovir in pediatric patients.
Contraindications
- Only for topical use of herpes labialis on the lips and face.
- DENAVIR should only be used on herpes labialis on the lips and face. Because no data are available, application to human mucous membranes is not recommended. Particular care should be taken to avoid application in or near the eyes since it may cause irritation. Lesions that do not improve or that worsen on therapy should be evaluated for secondary bacterial infection. The effect of DENAVIR has not been established in immunocompromised patients.
Warnings
|
ConditionName:
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- DENAVIR is contraindicated in patients with known hypersensitivity to the product or any of its components.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
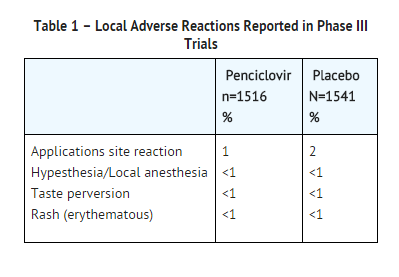
- In two double-blind, placebo-controlled trials, 1516 patients were treated with DENAVIR (penciclovir cream) and 1541 with placebo. One or more local adverse reactions were reported by 3% of the patients treated with DENAVIR and 4% of placebo-treated patients. The rates of reported local adverse reactions are shown in Table 1.
- Two studies, enrolling 108 healthy subjects, were conducted to evaluate the dermal tolerance of 5% penciclovir cream (a 5-fold higher concentration than the commercial formulation) compared to vehicle using repeated occluded patch testing methodology. The 5% penciclovir cream induced mild erythema in approximately one-half of the subjects exposed, an irritancy profile similar to the vehicle control in terms of severity and proportion of subjects with a response. No evidence of sensitization was observed.
- The following adverse reactions have been identified during post-approval use of DENAVIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The following events have been identified from worldwide post-marketing use of DENAVIR in treatment of recurrent herpes labialis (cold sores) in adults. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to DENAVIR.
- Skin: Aggravated condition, decreased therapeutic response, local edema, pain, paresthesia, pruritus, skin discoloration, and urticaria.
Adverse Reactions
Clinical Trials Experience
- One or more local skin reactions were reported by 3% of the patients treated with DENAVIR and 4% of placebo-treated patients.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Penciclovir in the drug label.
Body as a Whole
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- No drug interaction studies have been performed with DENAVIR. Due to minimal systemic absorption of DENAVIR, systemic drug interactions are unlikely.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category B
Category B
- There are no adequate and well-controlled studies in pregnant women.
Animal Data
- No adverse effects on the course and outcome of pregnancy or on fetal development were noted in rats and rabbits following the intravenous administration of penciclovir at doses of 80 and 60 mg/kg/day, respectively (estimated human equivalent doses of 13 and 18 mg/kg/day for the rat and rabbit, respectively, based on body surface area conversion; the body surface area doses being 260 and 355x the maximum recommended dose following topical application of the penciclovir cream). Because animal reproduction studies are not always predictive of human response, penciclovir should be used during pregnancy only if clearly needed.
- There is no information on whether penciclovir is excreted in human milk after topical administration. However, following oral administration of famciclovir (the oral prodrug of penciclovir) to lactating rats, penciclovir was excreted in breast milk at concentrations higher than those seen in the plasma. Therefore, a decision should be made whether to discontinue the drug, taking into account the importance of the drug to the mother.
- There are no data on the safety of penciclovir in newborns.
An open-label, uncontrolled trial with penciclovir cream 1% was conducted in 102 patients, ages 12-17 years, with recurrent herpes labialis. The frequency of adverse events was generally similar to the frequency previously reported for adult patients. Safety and effectiveness in pediatric patients less than 12 years of age have not been established.
In 74 patients ≥ 65 years of age, the adverse events profile was comparable to that observed in younger patients.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Penciclovir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Penciclovir during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Penciclovir with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Penciclovir with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Penciclovir with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Penciclovir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Penciclovir with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Penciclovir in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Penciclovir in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Penciclovir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Penciclovir in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Penciclovir in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Penciclovir in the drug label.
Overdosage
- Since penciclovir is poorly absorbed following oral administration, adverse reactions related to penciclovir ingestion are unlikely. There is no information on overdose.
Pharmacology
There is limited information regarding Penciclovir Pharmacology in the drug label.
Mechanism of Action
Structure
- DENAVIR (penciclovir) cream 1% contains penciclovir, an antiviral agent active against herpes viruses. DENAVIR is available for topical administration as a 1% white cream. Each gram of DENAVIR contains 10 mg of penciclovir and the following inactive ingredients: cetostearyl alcohol, mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water and white petrolatum.
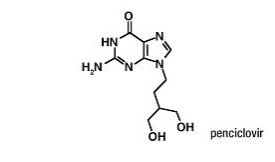
Figure 1: Structural Formula of Penciclovir
Penciclovir is a white to pale yellow solid. At 20°C it has a solubility of 0.2 mg/mL in methanol, 1.3 mg/mL in propylene glycol, and 1.7 mg/mL in water. In aqueous buffer (pH 2) the solubility is 10.0 mg/mL. Penciclovir is not hygroscopic. Its partition coefficient in n-octanol/water at pH 7.5 is 0.024 (logP = -1.62). Chemically, penciclovir is known as 9-[4-hydroxy-3-(hydroxymethyl)butyl] guanine. Its molecular formula is C10H15N5O3; its molecular weight is 253.26. It is a synthetic acyclic guanine derivative and has the following structure:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Penciclovir in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Penciclovir in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Penciclovir in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Penciclovir in the drug label.
How Supplied
Storage
There is limited information regarding Penciclovir Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Penciclovir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Penciclovir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Penciclovir in the drug label.
Precautions with Alcohol
- Alcohol-Penciclovir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Penciclovir
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Penciclovir |Label Name=Penciclovir11.png
}}
{{#subobject:
|Label Page=Penciclovir |Label Name=Penciclovir11.png
}}