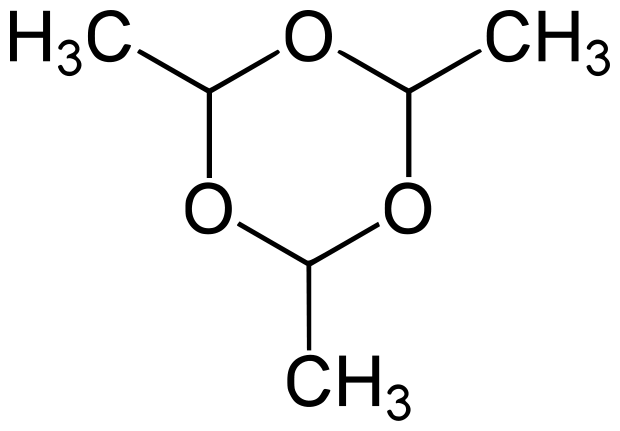
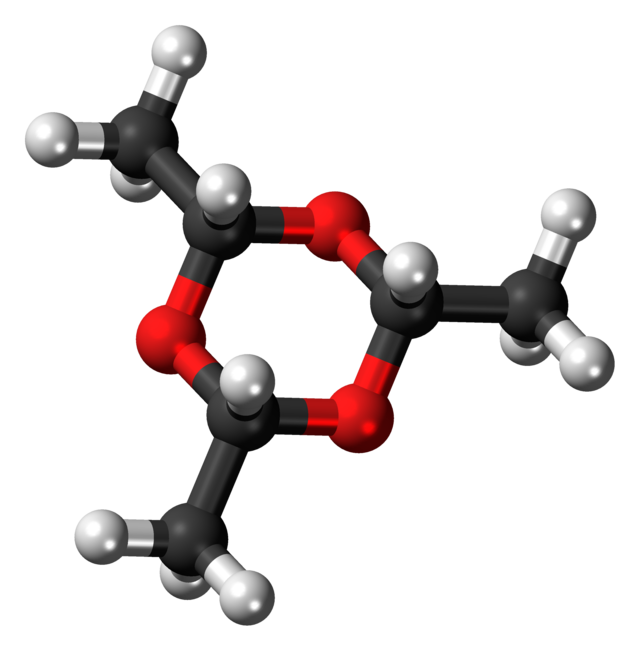
Paraldehyde
| |
| |
| Names | |
|---|---|
| IUPAC name
2,4,6-trimethyl-1,3,5-trioxane
| |
| Systematic IUPAC name
2,4,6-trimethyl-1,3,5-trioxane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| MeSH | Paraldehyde |
| UNII | |
| |
| |
| Properties | |
| C6H12O3 | |
| Molar mass | 132.16 g·mol−1 |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Paraldehyde |
|
Articles |
|---|
|
Most recent articles on Paraldehyde Most cited articles on Paraldehyde |
|
Media |
|
Powerpoint slides on Paraldehyde |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Paraldehyde at Clinical Trials.gov Clinical Trials on Paraldehyde at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Paraldehyde
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Paraldehyde Discussion groups on Paraldehyde Patient Handouts on Paraldehyde Directions to Hospitals Treating Paraldehyde Risk calculators and risk factors for Paraldehyde
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Paraldehyde |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Paraldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in alcohol. Paraldehyde slowly oxidizes in air, turning brown and producing an odour of acetic acid. It quickly reacts with most plastics and rubber.
Paraldehyde was first synthesized in 1829 by Wildenbusch.[2] It has uses in industry and medicine.
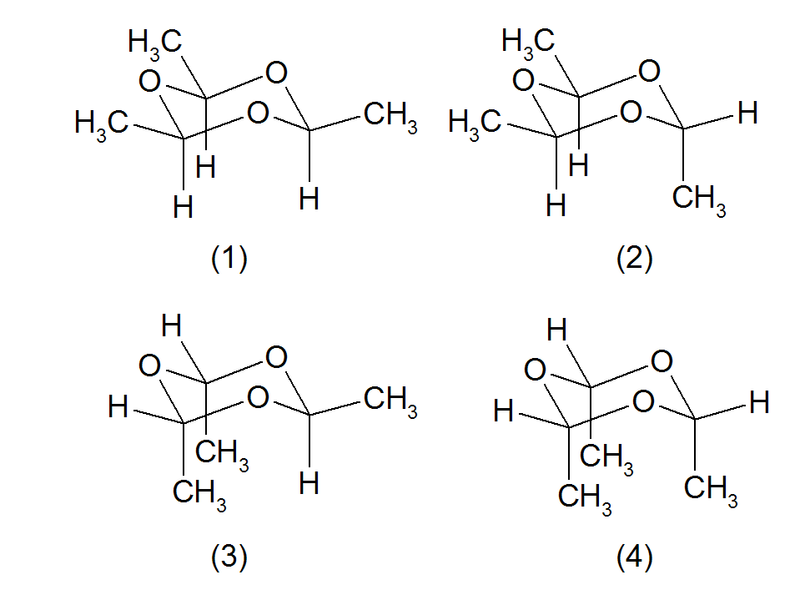
Stereochemistry
Theoretically four stereoisomeric structures are possible. The structures (1) and (2) are known as cis- and trans-paraldehyde. The structures (3) (a conformer of (2)) and (4) (a conformer of (1)) don't exist for steric reasons.[3][4]
Reactions
Heated with catalytic amounts of acid, it depolymerizes back to acetaldehyde:[5][6]
- C3H12O3 → 3CH3CHO
Since paraldehyde has better handling characteristics, it may be used indirectly or directly as a synthetic equivalent of anhydrous acetaldehyde (b.p. 20 °C). For example, it is used as-is in the synthesis of bromal (tribromoacetaldehyde):[7]
- C3H12O3 + 9 Br2 → 3 CBr3CHO + 9 HBr
Medical applications
Paraldehyde was introduced into clinical practice in the UK by the Italian physician Vincenzo Cervello in 1882.[2]
It is a central nervous system depressant and was soon found to be an effective anticonvulsant, hypnotic and sedative. It was included in some cough medicines as an expectorant (though there is no known mechanism for this function beyond the placebo effect).
Paraldehyde was the last injection given to Edith Alice Morrell in 1950 by the suspected serial killer John Bodkin Adams. He was tried for her murder but acquitted.
As a hypnotic/sedative
It was commonly used to induce sleep in sufferers from delirium tremens but has been replaced by other drugs in this regard. It is one of the safest hypnotics and was regularly given at bedtime in psychiatric hospitals and geriatric wards up to the 1960s. Up to 30% of the dose is excreted via the lungs (the rest via the liver). This contributes to a strong unpleasant odour on the breath.
As anti-seizure
It has been used in the treatment of convulsions.[8]
Today, paraldehyde is sometimes used to treat status epilepticus. Unlike diazepam and other benzodiazepines, it does not suppress breathing at therapeutic doses and so is safer when no resuscitation facilities exist or when the patient's breathing is already compromised.[9] This makes it a useful emergency medication for parents and other caretakers of children with epilepsy. Since the dose margin between the anticonvulsant and hypnotic effect is small, paraldehyde treatment usually results in sleep.
Administration

Generic paraldehyde is available in 5ml sealed glass ampoules. Production in the US has been discontinued, but it was previously marketed as Paral.
Paraldehyde has been given orally, rectally, intravenously and by intramuscular injection. It reacts with rubber and plastic which limits the time it may safely be kept in contact with some syringes or tubing before administration.
- Injection. Intramuscular injection can be very painful and lead to sterile abscesses, nerve damage, and tissue necrosis. Intravenous administration can lead to pulmonary edema, circulatory collapse and other complications.
- Oral. Paraldehyde has a hot burning taste and can upset the stomach. It is often mixed with milk or fruit juice in a glass cup and stirred with a metal spoon.
- Rectal. It may be mixed 1 part paraldehyde with 9 parts saline or, alternatively, with an equal mixture of peanut or olive oil.
Industrial applications
Paraldehyde is used in resin manufacture, as a preservative, and in other processes as a solvent.
It has been used in the generation of aldehyde fuchsin.[10]
References
- ↑ 1.0 1.1 "Oxford MSDS". Msds.chem.ox.ac.uk. Retrieved 2013-11-29.
- ↑ 2.0 2.1 López-Muñoz F, Ucha-Udabe R, Alamo C (December 2005). "The history of barbiturates a century after their clinical introduction". Neuropsychiatric disease and treatment. 1 (4): 329–43. PMC 2424120. PMID 18568113.
- ↑ Kewley, R.: Microwave spectrum of paraldehyde in Can. J. Chem. 48 (1970), 852–855
- ↑ Carpenter, D.C., Brockway, L.O.: The Electron Diffration Study of Paraldehyde in J. Amer. Chem. Soc. 58 (1936), 1270–1273
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Townend W, Mackway-Jones K (January 2002). "Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Phenytoin or paraldehyde as the second drug for convulsions in children". Emergency medicine journal : EMJ. 19 (1): 50. doi:10.1136/emj.19.1.50. PMC 1725762. PMID 11777879.
- ↑ Norris E, Marzouk O, Nunn A, McIntyre J, Choonara I (1999). "Respiratory depression in children receiving diazepam for acute seizures: a prospective study". Dev Med Child Neurol. 41 (5): 340–3. doi:10.1017/S0012162299000742. PMID 10378761.
- ↑ Nettleton GS (February 1982). "The role of paraldehyde in the rapid preparation of aldehyde fuchsin". The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 30 (2): 175–8. doi:10.1177/30.2.6174561. PMID 6174561.
External links
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Chemicals without a PubChem CID
- Articles without KEGG source
- Chemical articles with unknown parameter in Chembox
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Chembox having DSD data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Acetals
- Anticonvulsants
- Hypnotics
- Solvents