Pancuronium
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Black Box warning
See full prescribing information for complete Boxed Warning.
Condition Name: THIS DRUG SHOULD BE ADMINISTERED BY ADEQUATELY TRAINED INDIVIDUALS FAMILIAR WITH ITS ACTIONS, CHARACTERISTICS, AND HAZARDS.
|
Overview
Pancuronium is a skeletal muscle relaxant and neuromuscular blocking drugs that is FDA approved for the {{{indicationType}}} of general anesthesia; adjunct. There is a Black Box Warning for this drug as shown here. Common adverse reactions include musculoskeletal: prolonged neuromuscular block.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Dosage must be individualized
- General anesthesia; Adjunct: initial, 0.04 to 0.1 mg/kg IV (endotracheal intubation, 0.06 to 0.1 mg/kg IV); later incremental doses starting at 0.01 mg/kg may be used
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Induction of neuromuscular blockade - Percutaneous fetal procedure
Spasmodic torticollis
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pancuronium in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage must be individualized General anesthesia; Adjunct: (all ages except neonates) initial, 0.04 to 0.1 mg/kg IV (endotracheal intubation, 0.06 to 0.1 mg/kg IV); later incremental doses starting at 0.01 mg/kg may be used [2] General anesthesia; Adjunct: (neonates) administer a test dose of 0.02 mg/kg IV to measure responsiveness
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pancuronium in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pancuronium in pediatric patients.
Contraindications
Pancuronium Bromide Injection is contraindicated in patients known to be hypersensitive to the drug.
Warnings
|
Black Box warning
See full prescribing information for complete Boxed Warning.
Condition Name: THIS DRUG SHOULD BE ADMINISTERED BY ADEQUATELY TRAINED INDIVIDUALS FAMILIAR WITH ITS ACTIONS, CHARACTERISTICS, AND HAZARDS.
|
PANCURONIUM BROMIDE INJECTION SHOULD BE ADMINISTERED IN CAREFULLY ADJUSTED DOSES BY OR UNDER THE SUPERVISION OF EXPERIENCED CLINICIANS WHO ARE FAMILIAR WITH ITS ACTIONS AND THE POSSIBLE COMPLICATIONS THAT MIGHT OCCUR FOLLOWING ITS USE. THE DRUG SHOULD NOT BE ADMINISTERED UNLESS FACILITIES FOR INTUBATION, ARTIFICIAL RESPIRATION, OXYGEN THERAPY, AND REVERSAL AGENTS ARE IMMEDIATELY AVAILABLE. THE CLINICIAN MUST BE PREPARED TO ASSIST OR CONTROL RESPIRATION.
Anaphylaxis
Severe anaphylactic reactions to neuromuscular blocking agents, including pancuronium bromide, have been reported. These reactions have in some cases been life-threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should be taken in those individuals who have had previous anaphylactic reactions to other neuromuscular blocking agents since cross-reactivity between neuromuscular blocking agents, both depolarizing and non-depolarizing, has been reported in this class of drugs.
In patients who are known to have myasthenia gravis or the myasthenic (Eaton-Lambert) syndrome, small doses of pancuronium bromide may have profound effects. In such patients, a peripheral nerve stimulator and use of a small test dose may be of value in monitoring the response to administration of muscle relaxants.
Benzyl alcohol has been reported to be associated with a fatal “gasping syndrome” in premature infants.
Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to those received in flush solutions containing benzyl alcohol. Administration of high dosages of medications (including pancuronium) containing this preservative must take into account the total amount of benzyl alcohol administered. The recommended dosage range of pancuronium bromide for preterm and term infants includes amounts of benzyl alcohol well below that associated with toxicity; however, the amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources.
Adverse Reactions
Clinical Trials Experience
Neuromuscular
The most frequent adverse reaction to nondepolarizing blocking agents as a class consists of an extension of the drug’s pharmacological action beyond the time period needed. This may vary from skeletal muscle weakness to profound and prolonged skeletal muscle paralysis resulting in respiratory insufficiency or apnea. (See PRECAUTIONS: Pediatric Use).
Inadequate reversal of the neuromuscular blockade is possible with pancuronium bromide as with all curariform drugs. These adverse experiences are managed by manual or mechanical ventilation until recovery is judged adequate.
Prolonged paralysis and/or skeletal muscle weakness have been reported after long-term use to support mechanical ventilation in the intensive care unit.
Cardiovascular
See discussion of circulatory effects in CLINICAL PHARMACOLOGY.
Gastrointestinal
Salivation is sometimes noted during very light anesthesia, especially if no anticholinergic premedication is used.
Skin
An occasional transient rash is noted accompanying the use of pancuronium bromide.
Other
Although histamine release is not a characteristic action of pancuronium bromide, rare hypersensitivity reactions such as bronchospasm, flushing, redness, hypotension, tachycardia and other reactions possibly mediated by histamine release have been reported.
There have been post-marketing reports of severe allergic reactions (anaphylactic and anaphylactoid reactions) associated with use of neuromuscular blocking agents, including pancuronium bromide. These reactions, in some cases, have been life threatening and fatal. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency (see WARNINGS and PRECAUTIONS).
Postmarketing Experience
There is limited information regarding Pancuronium Postmarketing Experience in the drug label.
Drug Interactions
Prior administration of succinylcholine may enhance the neuromuscular blocking effect of pancuronium and increase its duration of action. If succinylcholine is used before pancuronium bromide, the administration of pancuronium bromide should be delayed until the patient starts recovering from succinylcholine-induced neuromuscular blockade.
If a small dose of pancuronium bromide is given at least 3 minutes prior to the administration of succinylcholine, in order to reduce the incidence and intensity of succinylcholine-induced fasciculations, this dose may induce a degree of neuromuscular block sufficient to cause respiratory depression in some patients.
Other nondepolarizing neuromuscular blocking agents (vecuronium, atracurium, d-tubocurarine, metocurine, and gallamine) behave in a clinically similar fashion to pancuronium bromide. The combination of pancuronium bromide-metocurine and pancuronium bromide-d-tubocurarine are significantly more potent than the additive effects of each of the individual drugs given alone, however, the duration of blockade of these combinations is not prolonged. There are insufficient data to support concomitant use of pancuronium and the other three above mentioned muscle relaxants in the same patient.
Inhalational Anesthetics
Use of volatile inhalational anesthetics such as enflurane, isoflurane, and halothane with pancuronium bromide will enhance neuromuscular blockade. Potentiation is most prominent with use of enflurane and isoflurane.
With the above agents, the intubating dose of pancuronium bromide may be the same as with balanced anesthesia unless the inhalational anesthetic has been administered for a sufficient time at a sufficient dose to have reached clinical equilibrium. The relatively long duration of action of pancuronium should be taken into consideration when the drug is selected for intubation in these circumstances.
Clinical experience and animal experiments suggest that pancuronium should be given with caution to patients receiving chronic tricyclic antidepressant therapy who are anesthetized with halothane because severe ventricular arrhythmias may result from this combination. The severity of the arrhythmias appear in part related to the dose of pancuronium.
Antibiotics
Parenteral/intraperitoneal administration of high doses of certain antibiotics may intensify or produce neuromuscular block on their own. The following antibiotics have been associated with various degrees of paralysis: aminoglycosides (such as neomycin, streptomycin, kanamycin, gentamicin, and dihydrostreptomycin); tetracyclines; bacitracin; polymyxin B; colistin; and sodium colistimethate. If these or other newly introduced antibiotics are used preoperatively or in conjunction with pancuronium bromide, unexpected prolongation of neuromuscular block should be considered a possibility.
Other
Experience concerning injection of quinidine during recovery from use of other muscle relaxants suggests that recurrent paralysis may occur. This possibility must also be considered for pancuronium bromide.
Electrolyte imbalance and diseases which lead to electrolyte imbalance, such as adrenal cortical insufficiency, have been shown to alter neuromuscular blockade. Depending on the nature of the imbalance, either enhancement or inhibition may be expected. Magnesium salts, administered for the management of toxemia of pregnancy, may enhance the neuromuscular blockade.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Animal reproduction studies have not been performed. It is not known whether pancuronium bromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pancuronium bromide should be given to a pregnant woman only if the administering clinician decides that the benefits outweigh the risks.
Pancuronium bromide may be used in operative obstetrics (Caesarean Section), but reversal of pancuronium may be unsatisfactory in patients receiving magnesium sulfate for toxemia of pregnancy because magnesium salts enhance neuromuscular blockade. Dosage should usually be reduced, as indicated, in such cases. It is also recommended that the interval between use of pancuronium and delivery be reasonably short to avoid clinically significant placental transfer.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pancuronium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pancuronium during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pancuronium in women who are nursing.
Pediatric Use
Dose response studies in children indicate that, with the exception of neonates, dosage requirements are the same as for adults. Neonates are especially sensitive to nondepolarizing neuromuscular blocking agents, such as pancuronium bromide, during the first month of life. It is recommended that a test dose of 0.02 mg/kg be given first in this group to measure responsiveness.
The prolonged use of pancuronium bromide for the management of neonates undergoing mechanical ventilation has been associated in rare cases with severe skeletal muscle weakness that may first be noted during attempts to wean such patients from the ventilator; such patients usually receive other drugs such as antibiotics which may enhance neuromuscular blockade. Microscopic changes consistent with disuse atrophy have been noted at autopsy. Although a cause-and-effect relationship has not been established, the benefits-to-risk ratio must be considered when there is a need for neuromuscular blockade to facilitate long-term mechanical ventilation of neonates.
Rare cases of unexplained, clinically significant methemoglobinemia have been reported in premature neonates undergoing emergency anesthesia and surgery which included combined use of pancuronium, fentanyl and atropine. A direct cause-and-effect relationship between the combined use of these drugs and the reported cases of methemoglobinemia has not been established
Geriatic Use
There is no FDA guidance on the use of Pancuronium in geriatric settings.
Gender
There is no FDA guidance on the use of Pancuronium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pancuronium with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pancuronium in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pancuronium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pancuronium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pancuronium in patients who are immunocompromised.
Administration and Monitoring
Administration
Pancuronium Bromide Injection is for intravenous use only. This drug should be administered by or under the supervision of experienced clinicians familiar with the use of neuromuscular blocking agents. DOSAGE MUST BE INDIVIDUALIZED IN EACH CASE. The dosage information which follows is derived from studies based upon units of drug per unit of body weight and is intended to serve as a guide only. Since potent inhalational anesthetics or prior use of succinylcholine may enhance the intensity and duration of pancuronium bromide (see PRECAUTIONS: Drug Interactions), the lower end of the recommended initial dosage range may suffice when pancuronium bromide is first used after intubation with succinylcholine and/or after maintenance doses of volatile liquid inhalational anesthetics are started. To obtain maximum clinical benefits of Pancuronium Bromide Injection and to minimize the possibility of overdosage, the monitoring of muscle twitch response to a peripheral nerve stimulator is advised.
In adults under balanced anesthesia the initial intravenous dosage range is 0.04 to 0.1 mg/kg. Later incremental doses starting at 0.01 mg/kg may be used. These increments slightly increase the magnitude of the blockade and significantly increase the duration of blockade because a significant number of myoneural junctions are still blocked when there is clinical need for more drug.
If Pancuronium Bromide Injection is used to provide skeletal muscle relaxation for endotracheal intubation, a bolus dose of 0.06 to 0.1 mg/kg is recommended. Conditions satisfactory for intubation are usually present within 2 to 3 minutes (see PRECAUTIONS).
Dosage in Children
Dose response studies in children indicate that, with the exception of neonates, dosage requirements are the same as for adults. Neonates are especially sensitive to nondepolarizing neuromuscular blocking agents, such as Pancuronium Bromide Injection, during the first month of life. It is recommended that a test dose of 0.02 mg/kg be given first in this group to measure responsiveness.
Caesarean Section
The dosage to provide relaxation for intubation and operation is the same as for general surgical procedures. The dosage to provide relaxation, following usage of succinylcholine for intubation (see PRECAUTIONS: Drug Interactions), is the same as for general surgical procedures.
Monitoring
There is limited information regarding Pancuronium Monitoring in the drug label.
IV Compatibility
Pancuronium Bromide Injection is compatible in solution with:
0.9% sodium chloride injection
5% dextrose injection
5% dextrose and sodium chloride injection
Lactated Ringer’s injection
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
When mixed with the above solutions in glass or plastic containers, Pancuronium Bromide Injection will remain stable in solution for 48 hours with no alteration in potency or pH; no decomposition is observed and there is no absorption to either the glass or plastic container.
Overdosage
The possibility of iatrogenic overdosage can be minimized by carefully monitoring the muscle twitch response to peripheral nerve stimulation.
Excessive doses of pancuronium bromide can be expected to produce enhanced pharmacological effects. Residual neuromuscular blockade beyond the time period needed may occur with pancuronium bromide as with other neuromuscular blockers. This may be manifested by skeletal muscle weakness, decreased respiratory reserve, low tidal volume, or apnea. A peripheral nerve stimulator may be used to assess the degree of residual neuromuscular blockade and help to differentiate residual neuromuscular blockade from other causes of decreased respiratory reserve.
Pyridostigmine bromide, neostigmine, or edrophonium, in conjunction with atropine or glycopyrrolate, will usually antagonize the skeletal muscle relaxant action of pancuronium bromide. Satisfactory reversal can be judged by adequacy of skeletal muscle tone and by adequacy of respiration. A peripheral nerve stimulator may also be used to monitor restoration of twitch response.
Failure of prompt reversal (within 30 minutes) may occur in the presence of extreme debilitation, carcinomatosis, and with concomitant use of certain broad spectrum antibiotics, or anesthetic agents and other drugs which enhance neuromuscular blockade or cause respiratory depression of their own. Under such circumstances, the management is the same as that of prolonged neuromuscular blockade. Ventilation must be supported by artificial means until the patient has resumed control of his respiration. Prior to the use of reversal agents, reference should be made to the specific package insert of the reversal agent.
Pharmacology
Mechanism of Action
Pancuronium bromide is a nondepolarizing neuromuscular blocking agent possessing all of the characteristic pharmacological actions of this class of drugs (curariform). It acts by competing for cholinergic receptors at the motor end-plate. The antagonism to acetylcholine is inhibited; and neuromuscular block is reversed by anticholinesterase agents such as pyridostigmine, neostigmine, and edrophonium. Pancuronium bromide is approximately 1/3 less potent than vecuronium and approximately 5 times as potent as d-tubocurarine: the duration of neuromuscular blockage produced by pancuronium bromide is longer than that of vecuronium at initially equipotent doses.
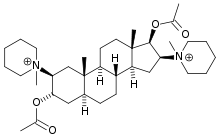
Structure
Pancuronium Bromide is a nondepolarizing neuromuscular blocking agent chemically designated as the aminosteroid 2β, 16β - dipiperidino-5α-androstane-3α, 17-β diol diacetate dimethobromide, C35H60Br2N2O4. It is a fine white odorless powder which is soluble in water, alcohol and chloroform.
It has the following structural formula:
Pancuronium Bromide Injection is available in sterile, isotonic, nonpyrogenic solution for injection. Each mL contains pancuronium bromide 1 mg; sodium acetate, anhydrous 1.2 mg; benzyl alcohol 10 mg as preservative. Sodium chloride added to adjust tonicity. May contain acetic acid and/or sodium hydroxide for pH adjustment. pH is 4.0 (3.8 to 4.2).
Pharmacodynamics
The ED95 (dose required to produce 95% suppression of muscle twitch response) is approximately 0.05 mg/kg under balanced anesthesia and 0.03 mg/kg under halothane anesthesia. These doses produce effective skeletal muscle relaxation (as judged by time from maximum effect to 25% recovery of control twitch height) for approximately 22 minutes; the duration from injection to 90% recovery of control twitch height is approximately 65 minutes. The intubating dose of 0.1 mg/kg (balanced anesthesia) will effectively abolish twitch response within approximately 4 minutes; time from injection to 25% recovery from this dose is approximately 100 minutes.
Supplemental doses to maintain muscle relaxation slightly increase the magnitude of block and significantly increase the duration of block. The use of a peripheral nerve stimulator is of benefit in assessing the degree of neuromuscular blockade.
The most characteristic circulatory effects of pancuronium, studied under halothane anesthesia, are a moderate rise in heart rate, mean arterial pressure and cardiac output; systemic vascular resistance is not changed significantly, and central venous pressure may fall slightly. The heart rate rise is inversely related to the rate immediately before administration of pancuronium, is blocked by prior administration of atropine, and appears unrelated to the concentration of halothane or dose of pancuronium.
Data on histamine assays and available clinical experience indicate that hypersensitivity reactions such as bronchospasm, flushing, redness, hypotension, tachycardia, and other reactions commonly associated with histamine release are rare. (See ADVERSE REACTIONS).
Pharmacokinetics
The elimination half-life of pancuronium has been reported to range between 89-161 minutes. The volume of distribution ranges from 241-280 mL/kg; and plasma clearance is approximately 1.1−1.9 mL/minute/kg. Approximately 40% of the total dose of pancuronium has been recovered in urine as unchanged pancuronium and its metabolites while approximately 11% has been recovered in bile. As much as 25% of an injected dose may be recovered as 3-hydroxy metabolite, which is half as potent a blocking agent as pancuronium. Less than 5% of the injected dose is recovered as 17-hydroxy metabolite and 3,17-dihydroxy metabolite, which have been judged to be approximately 50 times less potent than pancuronium. Pancuronium exhibits strong binding to gamma globulin and moderate binding to albumin. Approximately 13% is unbound to plasma protein. In patients with cirrhosis the volume of distribution is increased by approximately 50%, the plasma clearance is decreased by approximately 22%, and the elimination half-life is doubled. Similar results were noted in patients with biliary obstruction, except that plasma clearance was less than half the normal rate. The initial total dose to achieve adequate relaxation may, thus, be high in patients with hepatic and/or biliary tract dysfunction, while the duration of action is greater than usual.
The elimination half-life is doubled, and the plasma clearance is reduced by approximately 60% in patients with renal failure. The volume of distribution is variable, and in some cases elevated. The rate of recovery of neuromuscular blockade, as determined by peripheral nerve stimulation is variable and sometimes very much slower than normal.
Nonclinical Toxicology
There is limited information regarding Pancuronium Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pancuronium Clinical Studies in the drug label.
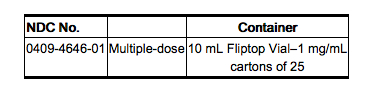
How Supplied
Pancuronium Bromide Injection is supplied as follows:
Storage
Store in refrigerator 2° to 8°C (36° to 46°F).
The 10 mL vial will maintain full clinical potency for up to six months at room temperature.
Revised: September, 2010
Printed in USA EN-2631
Hospira, Inc., Lake Forest, IL 60045 USA
Images
Drug Images
{{#ask: Page Name::Pancuronium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pancuronium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pancuronium Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pancuronium interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Pancuronium Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Pancuronium Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Pancuronium |Label Name=Pancuronium label.png
}}