Oxyphenisatine: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 49: | Line 49: | ||
}} | }} | ||
'''Oxyphenisatine''' (or '''oxyphenisatin''') is a [[laxative]].<ref>{{ cite journal | author = Farack, U. M.; Nell, G. | title = Mechanism of Action of Diphenolic Laxatives: The Role of Adenylate Cyclase and Mucosal Permeability | journal = Digestion | year = 1984 | volume = 30 | issue = 3 | pages = 191–194 | doi = 10.1159/000199105 | pmid = 6548720}}</ref> It is closely related to [[bisacodyl]], [[sodium picosulfate]], and [[phenolphthalein]]. Long term use is associated with liver damage,<ref>{{ cite journal | pmid = 6893676 | year = 1980 | last1 = Kotha, P.; Rake, M. O.; Willatt, D. | title = Liver Damage Induced by Oxyphenisatin | volume = 281 | issue = 6254 | pages = 1530 | pmc = 1714947 | journal = British Medical Journal | doi = 10.1136/bmj.281.6254.1530 | url = http://www.bmj.com/highwire/filestream/332472/field_highwire_article_pdf/0/1530.1.full.pdf | format = pdf }}</ref> and as a result, it was withdrawn in most countries in the early 1970s. The [[acetate]] [[derivative (chemistry)|derivative]] oxyphenisatine acetate was also once used as a laxative. | '''Oxyphenisatine''' (or '''oxyphenisatin''') is a [[laxative]].<ref>{{ cite journal | author = Farack, U. M.; Nell, G. | title = Mechanism of Action of Diphenolic Laxatives: The Role of Adenylate Cyclase and Mucosal Permeability | journal = Digestion | year = 1984 | volume = 30 | issue = 3 | pages = 191–194 | doi = 10.1159/000199105 | pmid = 6548720}}</ref> It is closely related to [[bisacodyl]], [[sodium picosulfate]], and [[phenolphthalein]]. Long term use is associated with liver damage,<ref>{{ cite journal | pmid = 6893676 | year = 1980 | last1 = Kotha, P.; Rake, M. O.; Willatt, D. | title = Liver Damage Induced by Oxyphenisatin | volume = 281 | issue = 6254 | pages = 1530 | pmc = 1714947 | journal = British Medical Journal | doi = 10.1136/bmj.281.6254.1530 | url = http://www.bmj.com/highwire/filestream/332472/field_highwire_article_pdf/0/1530.1.full.pdf | format = pdf }}</ref> and as a result, it was withdrawn in most countries in the early 1970s. The [[acetate]] [[derivative (chemistry)|derivative]] oxyphenisatine acetate was also once used as a laxative. | ||
Revision as of 12:50, 8 April 2015
Template:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox LogPTemplate:Chembox pKaTemplate:Chembox pKbTemplate:Chembox Pharmacology
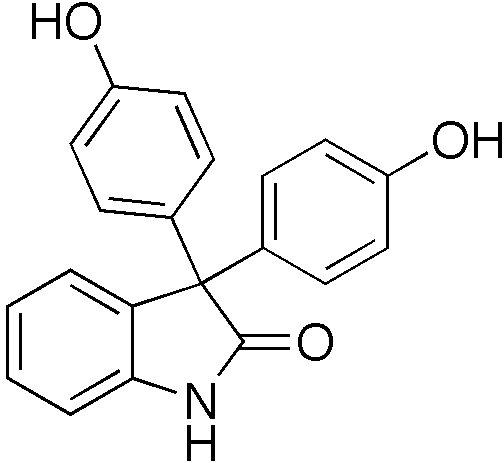
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,3-Bis(4-hydroxyphenyl)-1,3-dihydro-2H-indol-2-one[citation needed] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H15NO3 | |
| Molar mass | 317.34 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oxyphenisatine (or oxyphenisatin) is a laxative.[2] It is closely related to bisacodyl, sodium picosulfate, and phenolphthalein. Long term use is associated with liver damage,[3] and as a result, it was withdrawn in most countries in the early 1970s. The acetate derivative oxyphenisatine acetate was also once used as a laxative.
Natural chemical compounds similar to oxyphenisatine may be present in prunes,[4] but a recent review of the relevant scientific literature suggests that the laxative effect of prunes is due to other constituents including phenolic compounds (mainly neochlorogenic acids and chlorogenic acids) and sorbitol.[5]
References
- ↑ 1.0 1.1 1.2 SciFinder Scholar, version 2004.2; Chemical Abstracts Service, Registry Number 125-13-3, accessed September 1, 2011
- ↑ Farack, U. M.; Nell, G. (1984). "Mechanism of Action of Diphenolic Laxatives: The Role of Adenylate Cyclase and Mucosal Permeability". Digestion. 30 (3): 191–194. doi:10.1159/000199105. PMID 6548720.
- ↑ Kotha, P.; Rake, M. O.; Willatt, D. (1980). "Liver Damage Induced by Oxyphenisatin" (pdf). British Medical Journal. 281 (6254): 1530. doi:10.1136/bmj.281.6254.1530. PMC 1714947. PMID 6893676.
- ↑ Baum, H. M.; Sanders, R. G.; Straub, G. J. (1951). "The Occurrence of a Diphenyl Isatin in California Prunes". Journal of the American Pharmaceutical Association. 40 (7): 348–349. doi:10.1002/jps.3030400713. PMID 14850362.
- ↑ Stacewicz-Sapuntzakis, M.; Bowen, P. E.; Hussain, E. A.; Damayanti-Wood, B. I.; Farnsworth, N. R. (2001). "Chemical Composition and Potential Health Effects of Prunes: A Functional Food?". Critical Reviews in Food Science and Nutrition. 41 (4): 251–286. doi:10.1080/20014091091814. PMID 11401245.
Categories:
- Pages with script errors
- CS1 maint: Multiple names: authors list
- All articles with unsourced statements
- Articles with unsourced statements from August 2011
- Articles with invalid date parameter in template
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with unknown parameter in Chembox
- Articles with changed CASNo identifier
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Drug
- Lactams
- Indoles
- Phenols