Nicotine (inhalant): Difference between revisions
No edit summary |
No edit summary |
||
| Line 178: | Line 178: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=*Inhalant | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
Revision as of 16:23, 20 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nicotine (inhalant) is a Dependency Agent that is FDA approved for the treatment of Reducing the withdrawal symptoms, including nicotine craving, associated with quitting smoking. Common adverse reactions include skin irritation, nasal irritation, nasal spray, oral irritation, dizziness, headache, insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Reduces withdrawal symptoms, including nicotine craving, associated with quitting smoking
- Patients must desire to stop smoking and should be instructed to stop smoking completely as they begin using NICOTROL Inhaler. It is important that patients understand the instructions, and have their questions answered. They should clearly understand the directions for using the NICOTROL Inhaler and safely disposing of the used cartridges.
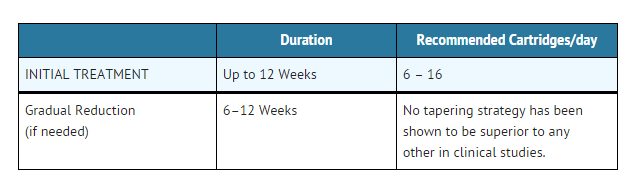
- The initial dosage of NICOTROL Inhaler is individualized. Patients may selftitrate to the level of nicotine they require. Most successful patients in the clinical trials used between 6 and 16 cartridges a day. Best effect was achieved by frequent continuous puffing (20 minutes). The recommended duration of treatment is 3 months, after which patients may be weaned from the NICOTROL Inhaler by gradual reduction of the daily dose over the following 6 to 12 weeks. The safety and efficacy of the continued use of NICOTROL Inhaler for periods longer than 6 months have not been studied and such use is not recommended.
- Dosing recommendations are summarized in the table below.
Directions to use
- Initial Treatment (Up to 12 Weeks)
- For best results, patients should be encouraged to use at least 6 cartridges per day at least for the first 3 to 6 weeks of treatment. In clinical trials, the average daily dose was >6 (range 3 to 18) cartridges for patients who successfully quit smoking. Additional doses may be needed to control the urge to smoke with a maximum of 16 cartridges daily for up to 12 weeks. Regular use of NICOTROL Inhaler during the first week of treatment may help patients adapt to the irritant effects of the product. Some patients may exhibit signs or symptoms of nicotine withdrawal or excess which will require an adjustment of the dosage (see INDIVIDUALIZATION OF DOSAGE).
- Gradual Reduction of Dose (Up to 12 Weeks)
- Most patients will need to gradually discontinue the use of NICOTROL Inhaler after the initial treatment period. Gradual reduction of dose may begin after twelve weeks of initial treatment and may last for up to twelve weeks. Recommended strategies for discontinuing use include suggesting to patients that they use the product less frequently, keep a tally of daily usage, try to meet a steadily reducing target or set a planned quit date for stopping use of the product.
- Individualization of Dosage
- The NICOTROL Inhaler provides the smoker with adequate amounts of nicotine to reduce the urge to smoke, and may provide some degree of comfort by providing a hand-to-mouth ritual similar to smoking, although the importance of such an effect in smoking cessation is, as yet, unknown.
- The success or failure of smoking cessation is influenced by the quality, intensity and frequency of supportive care. Patients are more likely to quit smoking if they are seen frequently and participate in formal smoking cessation programs.
- The goal of NICOTROL Inhaler therapy is complete abstinence. If a patient is unable to stop smoking by the fourth week of therapy, treatment should probably be discontinued.
Patients who fail to quit on any attempt may benefit from interventions to improve their chances for success on subsequent attempts. Patients who were unsuccessful should be counseled and should then probably be given a therapeutic holiday before the next attempt. A new quit attempt should be encouraged when conditions are more favorable.
- Based on the clinical trials, a reasonable approach to assisting patients in their attempt to quit smoking is to begin initial treatment, using the recommended dosage (See DOSAGE AND ADMINISTRATION). Dosage can then be adjusted in those patients with signs or symptoms of nicotine withdrawal or excess. Patients who are successfully abstinent on NICOTROL Inhaler should be treated at the selected dosage for up to 12 weeks, after which use of the Inhaler should be gradually reduced over the next 6 to 12 weeks. Some patients may not require gradual reduction of dosage and may abruptly stop treatment successfully. The safe use of this product for longer than 6 months has not been established.
- The symptoms of nicotine withdrawal overlap those of nicotine excess (See PHARMACODYNAMICS andADVERSE REACTIONS sections). Since patients using NICOTROL Inhaler may also smoke intermittently, it is sometimes difficult to determine if they are experiencing nicotine withdrawal or nicotine excess. Controlled clinical trials of nicotine products suggest that palpitations, nausea and sweating are more often symptoms of nicotine excess, whereas anxiety, nervousness and irritability are more often symptoms of nicotine withdrawal.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nicotine (inhalant) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nicotine (inhalant) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Nicotine (inhalant) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nicotine (inhalant) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nicotine (inhalant) in pediatric patients.
Contraindications
There is limited information regarding Nicotine (inhalant) Contraindications in the drug label.
Warnings
- If you are pregnant or breast-feeding,
Ask a doctor before use if you have
- a sodium-restricted diet
- heart disease, recent heart attack, or irregular heartbeat. Nicotine can increase your heart rate.
- high blood pressure not controlled with medication. Nicotine can increase your blood pressure.
- stomach ulcer or diabetes
- Ask a doctor or pharmacist before use if you are
- using a non-nicotine stop smoking drug
- taking prescription medicine for depression or asthma. Your prescription dose may need to be adjusted.
- Stop use and ask a doctor if
- mouth problems occur
- persistent indigestion or severe sore throat occurs
- irregular heartbeat or palpitations occur
- Keep out of reach of children and pets.
- Nicotine lozenges may have enough nicotine to make children and pets sick. If you need to remove the lozenge, wrap it in paper and throw away in the trash. In case of overdose, get medical help or contact a Poison Control Center right away.
Precautions
- General
- The patient should be urged to stop smoking completely when initiating NICOTROL Inhaler therapy (See DOSAGE AND ADMINISTRATION). Patients should be informed that if they continue to smoke while using the product, they may experience adverse effects due to peak nicotine levels higher than those experienced from smoking alone. If there is a clinically significant increase in cardiovascular or other effects attributable to nicotine, the treatment should be discontinued (See WARNINGS). Physicians should anticipate that concomitant medications may need dosage adjustment (See DRUG INTERACTIONS). Sustained use (beyond 6 months) of NICOTROL Inhaler by patients who stop smoking has not been studied and is not recommended. (See DRUG ABUSE AND DEPENDENCE).
- Bronchospastic Disease
- NICOTROL Inhaler has not been specifically studied in asthma or chronic pulmonary disease. Nicotine is an airway irritant and might cause bronchospasm. NICOTROL Inhaler should be used with caution in patients with bronchospastic disease. Other forms of nicotine replacement might be preferable in patients with severe bronchospastic airway disease.
- Cardiovascular or Peripheral Vascular Diseases
- The risks of nicotine replacement in patients with cardiovascular and peripheral vascular diseases should be weighed against the benefits of including nicotine replacement in a smoking cessation program for them. Specifically, patients with coronary heart disease (history of myocardial infarction and/or angina pectoris), serious cardiac arrhythmias, or vasospastic diseases (Buerger's disease, Prinzmetal's variant angina and Raynaud's phenomena) should be evaluated carefully before nicotine replacement is prescribed.
- Tachycardia and palpitations have been reported occasionally with the use of NICOTROL Inhaler as well as with other nicotine replacement therapies. No serious cardiovascular events were reported in clinical studies with NICOTROL Inhaler, but if such symptoms occur, its use should be discontinued.
- NICOTROL Inhaler generally should not be used in patients during the immediate post-myocardial infarction period, nor in patients with serious arrhythmias, or with severe or worsening angina.
- Renal or Hepatic Insufficiency
- The pharmacokinetics of nicotine have not been studied in the elderly or in patients with renal or hepatic impairment. However, given that nicotine is extensively metabolized and that its total system clearance is dependent on liver blood flow, some influence of hepatic impairment on drug kinetics (reduced clearance) should be anticipated. Only severe renal impairment would be expected to affect the clearance of nicotine or its metabolites from the circulation (See PHARMACOKINETICS).
- Endocrine Diseases
- NICOTROL Inhaler therapy should be used with caution in patients with hyperthyroidism, pheochromocytoma or insulin-dependent diabetes, since nicotine causes the release of catecholamines by the adrenal medulla.
- Peptic Ulcer Disease
- Nicotine delays healing in peptic ulcer disease; therefore, NICOTROL Inhaler therapy should be used with caution in patients with active peptic ulcers and only when the benefits of including nicotine replacement in a smoking cessation program outweigh the risks.
- Accelerated Hypertension
- Nicotine therapy constitutes a risk factor for development of malignant hypertension in patients with accelerated hypertension; therefore, NICOTROL Inhaler therapy should be used with caution in these patients and only when the benefits of including nicotine replacement in a smoking cessation program outweigh the risks.
Adverse Reactions
Clinical Trials Experience
- Assessment of adverse events in the 1,439 patients (730 on active drug) who participated in controlled clinical trials (including three dose finding studies) is complicated by the occurrence of signs and symptoms of nicotine withdrawal in some patients and nicotine excess in others. The incidence of adverse events is confounded by: (1) the many minor complaints that smokers commonly have, (2) continued smoking by many patients and (3) the local irritation from both the active drug and the placebo.
- Local Irritation
- NICOTROL Inhaler and the placebo were both associated with local irritant side effects. Local irritation in mouth and throat was reported by 40% of patients on active drug as compared to 18% of patients on placebo. Irritant effects were higher in the two pivotal trials with higher doses being 66% on active drug and 42% on placebo. Coughing (32% active versus 12% placebo) and rhinitis (23% active versus 16% placebo) were also higher on active drug. The majority of patients rated these symptoms as mild. The frequency of cough, and mouth and throat irritation declined with continued use of NICOTROL Inhaler. Other adverse events that occurred in over 3% of patients on active drug in placebo controlled pivotal trials considered possibly related to the local irritant effects of the NICOTROL Inhaler are taste comments, pain in jaw and neck, tooth disorders and sinusitis.
- Withdrawal
- Symptoms of withdrawal were common in both active and placebo groups. Common withdrawal symptoms seen in over 3% of patients on active drug included: dizziness, anxiety, sleep disorder, depression, withdrawal syndrome, drug dependence, fatigue and myalgia.
- Nicotine-Related Adverse Events
- The most common nicotine-related adverse event was dyspepsia. This was present in 18% of patients in the active group compared to 9% of patients in the placebo group. Other nicotine related events present in greater than 3% of patients on active drug include nausea, diarrhea, and hiccup.
- Smoking Related Adverse Events
- Smoking related adverse events present in greater than 3% of patients on active drug include chest discomfort, bronchitis, and hypertension.
- Other Adverse Events
- Adverse events of unknown relationship to nicotine occurring in greater than 3% of patients on active drug include headache (26% of patients on active and 15% of patients on placebo), influenza-like symptoms, pain, back-pain, allergy, paresthesias, flatulence and fever.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nicotine (inhalant) in the drug label.
Drug Interactions
- Physiological changes resulting from smoking cessation, with or without nicotine replacement, may alter the pharmacokinetics of certain concomitant medications, such as tricyclic antidepressants and theophylline. Doses of these and perhaps other medications may need to be adjusted in patients who successfully quit smoking.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
There is no FDA guidance on usage of Nicotine (inhalant) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nicotine (inhalant) in women who are pregnant.
Labor and Delivery
- NICOTROL Inhaler is not recommended for use during labor and delivery. The effect of nicotine on a mother or the fetus during labor is unknown.
Nursing Mothers
- Caution should be exercised when the NICOTROL Inhaler is administered to nursing mothers. The safety of NICOTROL Inhaler therapy in nursing infants has not been examined. Nicotine passes freely into breast milk; the milk to plasma ratio averages 2.9. Nicotine is absorbed orally. An infant has the ability to clear nicotine by hepatic first-pass clearance; however, the efficiency of removal is probably lowest at birth. Nicotine concentrations in milk can be expected to be lower with NICOTROL Inhaler when used as recommended than with cigarette smoking, as maternal plasma nicotine concentrations are generally reduced with nicotine replacement. The risk of exposure of the infant to nicotine from NICOTROL Inhaler therapy should be weighed against the risks associated with the infant's exposure to nicotine from continued smoking by the mother (passive smoke exposure and contamination of breast milk with other components of tobacco smoke) and from the NICOTROL Inhaler alone, or in combination with continued smoking.
Pediatric Use
- Safety and effectiveness in pediatric and adolescent patients below the age of 18 years have not been established for any nicotine replacement product. However, no specific medical risk is known or expected in nicotine dependent adolescents. NICOTROL Inhaler should be used for the treatment of tobacco dependence in the older adolescent only if the potential benefit justifies the potential risk.
Geriatic Use
- Clinical studies of NICOTROL Inhaler did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects. Other reports on clinical experience have not identified differences between older and younger patients. In general, dosage selection for an elderly patient should be cautious, usually starting at the low end of the dosage range reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease.
Gender
There is no FDA guidance on the use of Nicotine (inhalant) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nicotine (inhalant) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nicotine (inhalant) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nicotine (inhalant) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nicotine (inhalant) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nicotine (inhalant) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Inhalant
Monitoring
There is limited information regarding Monitoring of Nicotine (inhalant) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Nicotine (inhalant) in the drug label.
Overdosage
- Signs and Symptoms of Nicotine Toxicity
- Signs and symptoms of an overdose of the NICOTROL Inhaler would be expected to be the same as those of acute nicotine poisoning including: pallor, cold sweat, nausea, salivation, vomiting, abdominal pain, diarrhea, headache, dizziness, disturbed hearing and vision, tremor, mental confusion, and weakness. Prostration, hypotension, and respiratory failure may ensue with large overdoses. Lethal doses produce convulsions quickly and death follows as a result of peripheral or central respiratory paralysis or, less frequently, cardiac failure.
- Overdose from Inhalation
- The oral LD50 for nicotine is >5 mg/kg in dogs and >24 mg/kg in rodents. Death is due to respiratory paralysis. The oral minimum acute lethal dose for nicotine in adult humans is reported to be 40 to 60 mg (<1 mg/kg). The effects of using several cartridges in rapid succession are unknown (SeeWARNINGS, SAFETY NOTE CONCERNING CHILDREN).
- One cartridge of NICOTROL Inhaler contains 10 mg nicotine, of which, approximately 4 mg is delivered nicotine. It is unlikely that an excessive nicotine overdose will occur via inhalation. Should such an overdose occur, however, with signs of nicotine poisoning, the patient should be instructed to contact his/her physician immediately. For additional emergency information, call your regional poison center or call the National Capital Poison Center toll free (1-800-222-1222).
- Overdose from Ingestion
- Persons ingesting NICOTROL Inhaler cartridges should be referred to a health care facility for management. In unconscious patients with a secure airway, instill activated charcoal via a nasogastric tube. A saline cathartic or sorbitol may be added to the first dose of activated charcoal. Repeated doses of activated charcoal should be administered as long as the cartridge remains in the gastrointestinal tract since it will continue to release nicotine for many hours. The NICOTROL Inhaler cartridges can be identified with a radiogram.
- Management of Nicotine Poisoning
- Other supportive measures include diazepam or barbiturates for seizures, atropine for excessive bronchial secretions or diarrhea, respiratory support for respiratory failure, and vigorous fluid support for hypotension and cardiovascular collapse.
Pharmacology
There is limited information regarding Nicotine (inhalant) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Nicotine (inhalant) Mechanism of Action in the drug label.
Structure
There is limited information regarding Nicotine (inhalant) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nicotine (inhalant) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Nicotine (inhalant) in the drug label.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Nicotine itself does not appear to be a carcinogen in laboratory animals. However, nicotine and its metabolites increased the incidences of tumors in the cheek pouches of hamsters and forestomach of F344 rats, respectively when given in combination with tumor-initiators. One study, which could not be replicated, suggested that cotinine, the primary metabolite of nicotine, may cause lymphoreticular sarcoma in the large intestine of rats. Neither nicotine nor cotinine was mutagenic in the Ames salmonella test. Nicotine induced reparable DNA damage in an E. coli test system. Nicotine was shown to be genotoxic in a test system using Chinese hamster ovary cells. In rats and rabbits, implantation can be delayed or inhibited by a reduction in DNA synthesis that appears to be caused by nicotine. Studies have shown a decrease in litter size in rats treated with nicotine during gestation.
Clinical Studies
There is limited information regarding Clinical Studies of Nicotine (inhalant) in the drug label.
How Supplied
- Disposal
- See patient information sheet for instructions on handling and disposal. After using the NICOTROL Inhaler, carefully separate the mouthpiece, remove the used cartridge and throw it away, out of the reach of children and pets. Store the mouthpiece in the plastic storage case for further use. The mouthpiece is reusable and should be cleaned regularly with soap and water. The NICOTROL Inhaler cartridges can be detected on a radiogram.
How Supplied
- NICOTROL INHALER (nicotine inhalation system) is supplied as 168 cartridges each containing 10 mg (4 mg is delivered) nicotine (NDC 0009-5400-01). Each unit consists of 5 mouthpieces, 28 storage trays each containing 6 cartridges and 1 plastic storage case. A patient information leaflet is enclosed with the package.
- Store at room temperature not to exceed 77°F (25°C).
- Protect cartridges from light.
Storage
There is limited information regarding Nicotine (inhalant) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nicotine (inhalant) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nicotine (inhalant) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- A patient information sheet is included in the package of NICOTROL Inhaler cartridges dispensed to the patient. Patients should be encouraged to read the information sheet carefully and to ask their physician and pharmacist about the proper use of the product (See DOSAGE AND ADMINISTRATION). Patients must be advised to keep both used and unused cartridges out of the reach of children and pets.
Precautions with Alcohol
- Alcohol-Nicotine (inhalant) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nicotine (inhalant) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Nicotine (inhalant) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Nicotine (inhalant) |Label Name=Nicotine (inhalant)04.png
}}
{{#subobject:
|Label Page=Nicotine (inhalant) |Label Name=Nicotine (inhalant)05.png
}}