Natalizumab: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{GP}} | |||
|genericName=Natalizumab | |||
|aOrAn=a | |||
|drugClass=monoclonal antibody | |||
|indicationType=treatment | |||
|indication=multiple sclerosis (MS) and Crohn's disease (CD) | |||
|hasBlackBoxWarning=Yes | |||
|adverseReactions=headache, fatigue, arthralgia, urinary tract infection, lower respiratory tract infection, gastroenteritis, vaginitis, depression, pain in extremity, abdominal discomfort, diarrhea NOS, and rash | |||
|blackBoxWarningTitle=WARNING: PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY | |||
|blackBoxWarningBody=Natalizumab increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability. Risk factors for the development of PML include duration of therapy, prior use of immunosuppressants, and presence of anti-JCV antibodies. These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab. | |||
Healthcare professionals should monitor patients on natalizumab for any new sign or symptom that may be suggestive of PML. TYSABRI dosing should be withheld immediately at the first sign or symptom suggestive of PML. For diagnosis, an evaluation that includes a gadolinium-enhanced magnetic resonance imaging (MRI) scan of the brain and, when indicated, cerebrospinal fluid analysis for JC viral DNA are recommended. | |||
Because of the risk of PML, natalizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the TOUCH Prescribing Program. | |||
|fdaLIADAdult=====Multiple Sclerosis (MS)==== | |||
* Is indicated as monotherapy for the treatment of patients with relapsing forms of multiple sclerosis. | |||
* Natalizumab increases the risk of PML. | |||
* When initiating and continuing treatment with natalizumab, physicians should consider whether the expected benefit of natalizumab is sufficient to offset this risk. | |||
* Dosage: | |||
:* 300 mg intravenous infusion over one hour every four weeks. | |||
====Crohn's Disease (CD)==== | |||
* Is indicated for inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn's disease with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional CD therapies and inhibitors of TNF-α. | |||
* Natalizumab should not be used in combination with immunosuppressants (e.g., 6-mercaptopurine, azathioprine, cyclosporine, or methotrexate) or inhibitors of TNF-α. | |||
* Dosage: | |||
:* 300 mg intravenous infusion over one hour every four weeks | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Natalizumab in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Natalizumab in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Natalizumab in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Natalizumab in pediatric patients. | |||
|contraindications=* TYSABRI is contraindicated in patients who have or have had progressive multifocal leukoencephalopathy (PML). | |||
* TYSABRI should not be administered to a patient who has had a hypersensitivity reaction to TYSABRI. Observed reactions range from urticaria to anaphylaxis | |||
|warnings=====Progressive Multifocal Leukoencephalopathy==== | |||
Progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability, has occurred in patients who have received TYSABRI. | |||
Three factors that are known to increase the risk of PML in TYSABRI-treated patients have been identified: | |||
* Longer treatment duration, especially beyond 2 years. There is limited experience in patients who have received more than 6 years of TYSABRI treatment. | |||
* Prior treatment with an immunosuppressant (e.g., mitoxantrone, azathioprine, methotrexate, cyclophosphamide, mycophenolate mofetil). | |||
* The presence of anti-JCV antibodies. Patients who are anti-JCV antibody positive have a higher risk for developing PML. | |||
These factors should be considered in the context of expected benefit when initiating and continuing treatment with TYSABRI. | |||
[[File:Natalizumab PML.png|thumb|none|600px]] | |||
Infection by the JC virus is required for the development of PML. Anti-JCV antibody testing should not be used to diagnose PML. Anti-JCV antibody negative status indicates that exposure to the JC virus has not been detected. Patients who are anti-JCV antibody negative have a lower risk of PML than those who are positive. Patients who are anti-JCV antibody negative are still at risk for the development of PML due to the potential for a new JCV infection or a false negative test result. The reported rate of seroconversion in patients with MS (changing from anti-JCV antibody negative to positive and remaining positive in subsequent testing) is 3 to 8 percent annually. In addition, some patients' serostatus may change intermittently. Therefore, patients with a negative anti-JCV antibody test result should be retested periodically. For purposes of risk assessment, a patient with a positive anti-JCV antibody test at any time is considered anti-JCV antibody positive regardless of the results of any prior or subsequent anti-JCV antibody testing. When assessed, anti-JCV antibody status should be determined using an analytically and clinically validated immunoassay. Anti-JCV antibody testing should not be performed for at least two weeks following plasma exchange due to the removal of antibodies from the serum. | |||
There are no known interventions that can reliably prevent PML or adequately treat PML if it occurs. It is not known whether early detection of PML and discontinuation of TYSABRI will mitigate the disease. PML has been reported following discontinuation of TYSABRI in patients who did not have findings suggestive of PML at the time of discontinuation. Patients should continue to be monitored for any new signs or symptoms that may be suggestive of PML for at least six months following discontinuation of TYSABRI. | |||
Ordinarily, patients receiving chronic immunosuppressant or immunomodulatory therapy or who have systemic medical conditions resulting in significantly compromised immune system function should not be treated with TYSABRI. | |||
Because of the risk of PML, TYSABRI is available only under a restricted distribution program, the TOUCH® Prescribing Program. | |||
In multiple sclerosis patients, an MRI scan should be obtained prior to initiating therapy with TYSABRI. This MRI may be helpful in differentiating subsequent multiple sclerosis symptoms from PML. | |||
In Crohn's disease patients, a baseline brain MRI may also be helpful to distinguish pre-existent lesions from newly developed lesions, but brain lesions at baseline that could cause diagnostic difficulty while on TYSABRI therapy are uncommon. | |||
Healthcare professionals should monitor patients on TYSABRI for any new sign or symptom suggestive of PML. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. The progression of deficits usually leads to death or severe disability over weeks or months. Withhold TYSABRI dosing immediately at the first sign or symptom suggestive of PML. | |||
For diagnosis of PML, an evaluation including a gadolinium-enhanced MRI scan of the brain and, when indicated, cerebrospinal fluid analysis for JC viral DNA are recommended. If the initial evaluations for PML are negative but clinical suspicion for PML remains, continue to withhold TYSABRI dosing and repeat the evaluations. | |||
There are no known interventions that can adequately treat PML if it occurs. Three sessions of plasma exchange over 5 to 8 days were shown to accelerate TYSABRI clearance in a study of 12 patients with MS who did not have PML, although in the majority of patients alpha-4 integrin receptor binding remained high. Adverse events which may occur during plasma exchange include clearance of other medications and volume shifts, which have the potential to lead to hypotension or pulmonary edema. Although plasma exchange has not been studied in TYSABRI treated patients with PML, it has been used in such patients in the postmarketing setting to remove TYSABRI more quickly from the circulation. Anti-JCV antibody testing should not be performed during or for at least two weeks following plasma exchange due to the removal of antibodies from the serum. | |||
Immune reconstitution inflammatory syndrome (IRIS) has been reported in the majority of TYSABRI treated patients who developed PML and subsequently discontinued TYSABRI. In almost all cases, IRIS occurred after plasma exchange was used to eliminate circulating TYSABRI. It presents as a clinical decline in the patient's condition after TYSABRI removal (and in some cases after apparent clinical improvement) that may be rapid, can lead to serious neurological complications or death and is often associated with characteristic changes in the MRI. TYSABRI has not been associated with IRIS in patients discontinuing treatment with TYSABRI for reasons unrelated to PML. In TYSABRI-treated patients with PML, IRIS has been reported within days to several weeks after plasma exchange. Monitoring for development of IRIS and appropriate treatment of the associated inflammation should be undertaken. | |||
====TYSABRI TOUCH Prescribing Program==== | |||
TYSABRI is available only through a restricted program under a REMS called the TOUCH® Prescribing Program because of the risk of PML. | |||
For prescribers and patients, the TOUCH® Prescribing Program has two components: MS TOUCH® (for patients with multiple sclerosis) and CD TOUCH® (for patients with Crohn's disease). | |||
Selected requirements of the TOUCH® Prescribing Program include the following: | |||
* Prescribers must be certified and comply with the following: | |||
:* Review the TOUCH Prescribing Program prescriber educational materials, including the full prescribing information. | |||
:* Educate patients on the benefits and risks of treatment with TYSABRI, ensure that patients receive the Medication Guide, and encourage them to ask questions. | |||
:* Review, complete, and sign the Patient-Prescriber Enrollment Form. | |||
:* Evaluate patients three months after the first infusion, six months after the first infusion, every six months thereafter, and for at least six months after discontinuing TYSABRI. | |||
:* Determine every six months whether patients should continue on treatment and, if so, authorize treatment for another six months. | |||
:* Submit to Biogen Idec the “TYSABRI Patient Status Report and Reauthorization Questionnaire” six months after initiating treatment and every six months thereafter. | |||
:* Complete an “Initial Discontinuation Questionnaire” when TYSABRI is discontinued and a “6-Month Discontinuation Questionnaire” following discontinuation of TYSABRI. | |||
:* Report cases of PML, hospitalizations due to opportunistic infections, or deaths to Biogen Idec at 1-800-456-2255 as soon as possible. | |||
* Patients must be enrolled in the TOUCH Prescribing Program, read the Medication Guide, understand the risks associated with TYSABRI and complete and sign the Patient-Prescriber Enrollment Form. | |||
* Pharmacies and infusion centers must be specially certified to dispense or infuse TYSABRI. | |||
====Herpes Encephalitis and Meningitis==== | |||
Natalizumab increases the risk of developing encephalitis and meningitis caused by herpes simplex and varicella zoster viruses. Serious, life-threatening, and sometimes fatal cases have been reported in the postmarketing setting in multiple sclerosis patients receiving TYSABRI. Laboratory confirmation in those cases was based on positive PCR for viral DNA in the cerebrospinal fluid. The duration of treatment with TYSABRI prior to onset ranged from a few months to several years. Monitor patients receiving TYSABRI for signs and symptoms of meningitis and encephalitis. If herpes encephalitis or meningitis occurs, TYSBARI should be discontinued, and appropriate treatment for herpes encephalitis/meningitis should be administered. | |||
====Hepatotoxicity==== | |||
Clinically significant liver injury, including acute liver failure requiring transplant, has been reported in patients treated with TYSABRI in the postmarketing setting. Signs of liver injury, including markedly elevated serum hepatic enzymes and elevated total bilirubin, occurred as early as six days after the first dose; signs of liver injury have also been reported for the first time after multiple doses. In some patients, liver injury recurred upon rechallenge, providing evidence that TYSABRI caused the injury. The combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury that may lead to death or the need for a liver transplant in some patients. | |||
Natalizumab should be discontinued in patients with jaundice or other evidence of significant liver injury (e.g., laboratory evidence). | |||
====Hypersensitivity/Antibody Formation==== | |||
Hypersensitivity reactions have occurred in patients receiving TYSABRI, including serious systemic reactions (e.g., anaphylaxis) which occurred at an incidence of <1%. These reactions usually occur within two hours of the start of the infusion. Symptoms associated with these reactions can include urticaria, dizziness, fever, rash, rigors, pruritus, nausea, flushing, hypotension, dyspnea, and chest pain. Generally, these reactions are associated with antibodies to TYSABRI. | |||
If a hypersensitivity reaction occurs, discontinue administration of TYSABRI and initiate appropriate therapy. Patients who experience a hypersensitivity reaction should not be re-treated with TYSABRI. Hypersensitivity reactions were more frequent in patients with antibodies to TYSABRI compared to patients who did not develop antibodies to TYSABRI in both MS and CD studies. Therefore, the possibility of antibodies to TYSABRI should be considered in patients who have hypersensitivity reactions. | |||
Antibody testing: If the presence of persistent antibodies is suspected, antibody testing should be performed. Antibodies may be detected and confirmed with sequential serum antibody tests. Antibodies detected early in the treatment course (e.g., within the first six months) may be transient and disappear with continued dosing. Repeat testing at three months after the initial positive result is recommended in patients in whom antibodies are detected to confirm that antibodies are persistent. Prescribers should consider the overall benefits and risks of TYSABRI in a patient with persistent antibodies. | |||
Experience with monoclonal antibodies, including TYSABRI, suggests that patients who receive therapeutic monoclonal antibodies after an extended period without treatment may be at higher risk of hypersensitivity reactions than patients who received regularly scheduled treatment. Given that patients with persistent antibodies to TYSABRI experience reduced efficacy, and that hypersensitivity reactions are more common in such patients, consideration should be given to testing for the presence of antibodies in patients who wish to recommence therapy following a dose interruption. Following a period of dose interruption, patients testing negative for antibodies prior to re-dosing have a risk of antibody development with re-treatment that is similar to TYSABRI naïve patients. | |||
====Immunosuppression/Infections==== | |||
The immune system effects of TYSABRI may increase the risk for infections. In Study MS1, certain types of infections, including pneumonias and urinary tract infections (including serious cases), gastroenteritis, vaginal infections, tooth infections, tonsillitis, and herpes infections, occurred more often in TYSABRI-treated patients than in placebo-treated patients. One opportunistic infection, a cryptosporidial gastroenteritis with a prolonged course, was observed in a patient who received TYSABRI in Study MS1. | |||
In Studies MS1 and MS2, an increase in infections was seen in patients concurrently receiving short courses of corticosteroids. However, the increase in infections in TYSABRI-treated patients who received steroids was similar to the increase in placebo-treated patients who received steroids. | |||
In CD clinical studies, opportunistic infections (pneumocystis carinii pneumonia, pulmonary mycobacterium avium intracellulare, bronchopulmonary aspergillosis, and burkholderia cepacia) have been observed in <1% of TYSABRI-treated patients; some of these patients were receiving concurrent immunosuppressants. | |||
In Studies CD1 and CD2, an increase in infections was seen in patients concurrently receiving corticosteroids. However, the increase in infections was similar in placebo-treated and TYSABRI-treated patients who received steroids. | |||
Concurrent use of antineoplastic, immunosuppressant, or immunomodulating agents may further increase the risk of infections, including PML and other opportunistic infections, over the risk observed with use of TYSABRI alone. The safety and efficacy of TYSABRI in combination with antineoplastic, immunosuppressant, or immunomodulating agents have not been established. Patients receiving chronic immunosuppressant or immunomodulatory therapy or who have systemic medical conditions resulting in significantly compromised immune system function should not ordinarily be treated with TYSABRI. The risk of PML is also increased in patients who have been treated with an immunosuppressant prior to receiving TYSABRI. | |||
For patients with Crohn's disease who start TYSABRI while on chronic corticosteroids, commence steroid withdrawal as soon as a therapeutic benefit has occurred. If the patient cannot discontinue systemic corticosteroids within six months, discontinue TYSABRI. | |||
====Laboratory Test Abnormalities==== | |||
In clinical trials, TYSABRI was observed to induce increases in circulating lymphocytes, monocytes, eosinophils, basophils, and nucleated red blood cells. Observed changes persisted during TYSABRI exposure, but were reversible, returning to baseline levels usually within 16 weeks after the last dose. Elevations of neutrophils were not observed. TYSABRI induces mild decreases in hemoglobin levels that are frequently transient. | |||
====Immunizations==== | |||
No data are available on the effects of vaccination in patients receiving TYSABRI. No data are available on the secondary transmission of infection by live vaccines in patients receiving TYSABRI. | |||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
The most common adverse reactions (incidence ≥ 10%) were headache and fatigue in both the multiple sclerosis (MS) and Crohn's disease (CD) studies. Other common adverse reactions (incidence ≥ 10%) in the MS population were arthralgia, urinary tract infection, lower respiratory tract infection, gastroenteritis, vaginitis, depression, pain in extremity, abdominal discomfort, diarrhea NOS, and rash. Other common adverse reactions (incidence ≥ 10%) in the CD population were upper respiratory tract infections and nausea. | |||
The most frequently reported adverse reactions resulting in clinical intervention (i.e., discontinuation of TYSABRI), in the MS studies were urticaria (1%) and other hypersensitivity reactions (1%), and in the CD studies (Studies CD1 and CD2) were the exacerbation of Crohn's disease (4.2%) and acute hypersensitivity reactions (1.5%). | |||
A total of 1617 multiple sclerosis patients in controlled studies received TYSABRI, with a median duration of exposure of 28 months. A total of 1563 patients received TYSABRI in all CD studies for a median exposure of 5 months; of these patients, 33% (n=518) received at least one year of treatment and 19% (n=297) received at least two years of treatment. | |||
====Multiple Sclerosis Clinical Studies==== | |||
The most frequently reported serious adverse reactions in Study MS1 [see Clinical Studies (14.1)] with TYSABRI were infections (3.2% versus 2.6% in placebo, including urinary tract infection [0.8% versus 0.3%] and pneumonia [0.6% versus 0%]), acute hypersensitivity reactions (1.1% versus 0.3%, including anaphylaxis/anaphylactoid reaction [0.8% versus 0%]), depression (1.0% versus 1.0%, including suicidal ideation or attempt [0.6% versus 0.3%]), and cholelithiasis (1.0% versus 0.3%). In Study MS2, serious adverse reactions of appendicitis were also more common in patients who received TYSABRI (0.8% versus 0.2% in placebo). | |||
TABLE 2 enumerates adverse reactions and selected laboratory abnormalities that occurred in Study MS1 at an incidence of at least 1 percentage point higher in TYSABRI-treated patients than was observed in placebo-treated patients. | |||
[[File:Natalizumab Adverse reactions in Study MS1.png|thumb|none|600px]] | |||
In Study MS2, peripheral edema was more common in patients who received TYSABRI (5% versus 1% in placebo). | |||
====Crohn's Disease Clinical Studies==== | |||
The following serious adverse events in the induction Studies CD1 and CD2 were reported more commonly with TYSABRI than placebo and occurred at an incidence of at least 0.3%: intestinal obstruction or stenosis (2% vs. 1% in placebo), acute hypersensitivity reactions (0.5% vs. 0%), abdominal adhesions (0.3% vs. 0%), and cholelithiasis (0.3% vs. 0%). Similar serious adverse events were seen in the maintenance Study CD3. TABLE 3 enumerates adverse drug reactions that occurred in Studies CD1 and CD2 (median exposure of 2.8 months). TABLE 4 enumerates adverse drug reactions that occurred in Study CD3 (median exposure of 11.0 months). | |||
[[File:Natalizumab Adverse reactions in Study CD1, CD2 and CD3.png|thumb|none|600px]] | |||
====Infections==== | |||
Progressive Multifocal Leukoencephalopathy (PML) occurred in three patients who received TYSABRI in clinical trials. Two cases of PML were observed in the 1869 patients with multiple sclerosis who were treated for a median of 120 weeks. These two patients had received TYSABRI in addition to interferon beta-1a. The third case occurred after eight doses in one of the 1043 patients with Crohn's disease who were evaluated for PML. In the postmarketing setting, additional cases of PML have been reported in TYSABRI-treated multiple sclerosis and Crohn's disease patients who were not receiving concomitant immunomodulatory therapy. | |||
In Studies MS1 and MS2, the rate of any type of infection was approximately 1.5 per patient-year in both TYSABRI-treated patients and placebo-treated patients. The infections were predominately upper respiratory tract infections, influenza, and urinary tract infections. In Study MS1, the incidence of serious infection was approximately 3% in TYSABRI-treated patients and placebo-treated patients. Most patients did not interrupt treatment with TYSABRI during infections. The only opportunistic infection in the multiple sclerosis clinical trials was a case of cryptosporidial gastroenteritis with a prolonged course. | |||
In Studies CD1 and CD2, the rate of any type of infection was 1.7 per patient-year in TYSABRI-treated patients and 1.4 per patient-year in placebo-treated patients. In Study CD3, the incidence of any type of infection was 1.7 per patient-year in TYSABRI-treated patients and was similar in placebo-treated patients. The most common infections were nasopharyngitis, upper respiratory tract infection, and influenza. The majority of patients did not interrupt TYSABRI therapy during infections and recovery occurred with appropriate treatment. Concurrent use of TYSABRI in CD clinical trials with chronic steroids and/or methotrexate, 6-MP, and azathioprine did not result in an increase in overall infections compared to TYSABRI alone; however, the concomitant use of such agents could lead to an increased risk of serious infections. | |||
In Studies CD1 and CD2, the incidence of serious infection was approximately 2.1% in both TYSABRI-treated patients and placebo-treated patients. In Study CD3, the incidence of serious infection was approximately 3.3% in TYSABRI-treated patients and approximately 2.8% in placebo-treated patients. | |||
In clinical studies for CD, opportunistic infections (pneumocystis carinii pneumonia, pulmonary mycobacterium avium intracellulare, bronchopulmonary aspergillosis, and burkholderia cepacia) have been observed in <1% of TYSABRI-treated patients; some of these patients were receiving concurrent immunosuppressants. Two serious non-bacterial meningitides occurred in TYSABRI-treated patients compared to none in placebo-treated patients. | |||
====Infusion-related Reactions==== | |||
An infusion-related reaction was defined in clinical trials as any adverse event occurring within two hours of the start of an infusion. In MS clinical trials, approximately 24% of TYSABRI-treated multiple sclerosis patients experienced an infusion-related reaction, compared to 18% of placebo-treated patients. In the controlled CD clinical trials, infusion-related reactions occurred in approximately 11% of patients treated with TYSABRI compared to 7% of placebo-treated patients. Reactions more common in the TYSABRI-treated MS patients compared to the placebo-treated MS patients included headache, dizziness, fatigue, urticaria, pruritus, and rigors. Acute urticaria was observed in approximately 2% of patients. Other hypersensitivity reactions were observed in 1% of patients receiving TYSABRI. Serious systemic hypersensitivity infusion reactions occurred in <1% of patients. All patients recovered with treatment and/or discontinuation of the infusion. | |||
Infusion-related reactions more common in CD patients receiving TYSABRI than those receiving placebo included headache, nausea, urticaria, pruritus, and flushing. Serious infusion reactions occurred in Studies CD1, CD2, and CD3 at an incidence of <1% in TYSABRI-treated patients. | |||
MS and CD patients who became persistently positive for antibodies to TYSABRI were more likely to have an infusion-related reaction than those who were antibody-negative. | |||
|drugInteractions=Because of the potential for increased risk of PML and other infections, Crohn's disease patients receiving TYSABRI should not be treated with concomitant immunosuppressants (e.g., 6-mercaptopurine, azathioprine, cyclosporine, or methotrexate) or inhibitors of TNF-α, and corticosteroids should be tapered in those patients with Crohn's disease who are on chronic corticosteroids when they start TYSABRI therapy. Ordinarily, MS patients receiving chronic immunosuppressant or immunomodulatory therapy should not be treated with TYSABRI. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Natalizumab has been shown to reduce pup survival in guinea pigs when given in doses 7 times the human dose, and has been shown to have hematologic effects on the fetus in monkeys when given in doses 2.3 times the human dose. There are no adequate and well-controlled studies in pregnant women. TYSABRI should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
|AUSPregCat=C | |||
|useInNursing=Natalizumab has been detected in human milk. The effects of this exposure on infants are unknown. | |||
|useInPed=Safety and effectiveness of TYSABRI in pediatric patients with multiple sclerosis or Crohn's disease below the age of 18 years have not been established. TYSABRI is not indicated for use in pediatric patients. | |||
|useInGeri=Clinical studies of TYSABRI did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. | |||
|useInRenalImpair=Pharmacokinetics of natalizumab in patients with renal or hepatic insufficiency have not been studied. | |||
|useInHepaticImpair=Pharmacokinetics of natalizumab in patients with renal or hepatic insufficiency have not been studied. | |||
|useInReproPotential=Natalizumab did not affect male fertility at doses up to 7-fold the clinical dose. | |||
|administration=Intravenous | |||
|overdose=Safety of doses higher than 300 mg has not been adequately evaluated. The maximum amount of TYSABRI that can be safely administered has not been determined. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 458283255 | |||
| image = | |||
<!--Monoclonal antibody data--> | |||
| type = mab | |||
| mab_type = mab | |||
| source = zu/o | |||
| target = alpha-4 [[integrin]] | |||
<!--Clinical data--> | |||
| tradename = Tysabri | |||
| Drugs.com = {{drugs.com|monograph|natalizumab}} | |||
| MedlinePlus = a605006 | |||
| licence_EU = Natalizumab | |||
| licence_US = Natalizumab | |||
| pregnancy_AU = C | |||
| pregnancy_US = C | |||
| legal_status = Rx-only | |||
| routes_of_administration = [[Intravenous infusion]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = n/a | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = 11 ± 4 days | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 189261-10-7 | |||
| ATC_prefix = L04 | |||
| ATC_suffix = AA23 | |||
| ATC_supplemental = | |||
| PubChem = | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00108 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 3JB47N2Q2P | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1201607 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = NA | |||
<!--Chemical data--> | |||
| chemical_formula = | |||
| molecular_weight = 149 [[Atomic mass unit|kDa]] | |||
}} | |||
|mechAction=Natalizumab binds to the α4-subunit of α4β1 and α4β7 integrins expressed on the surface of all leukocytes except neutrophils, and inhibits the α4-mediated adhesion of leukocytes to their counter-receptor(s). The receptors for the α4 family of integrins include vascular cell adhesion molecule-1 (VCAM-1), which is expressed on activated vascular endothelium, and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) present on vascular endothelial cells of the gastrointestinal tract. Disruption of these molecular interactions prevents transmigration of leukocytes across the endothelium into inflamed parenchymal tissue. In vitro, anti-α4-integrin antibodies also block α4-mediated cell binding to ligands such as osteopontin and an alternatively spliced domain of fibronectin, connecting segment-1 (CS-1). In vivo, natalizumab may further act to inhibit the interaction of α4-expressing leukocytes with their ligand(s) in the extracellular matrix and on parenchymal cells, thereby inhibiting further recruitment and inflammatory activity of activated immune cells. | |||
The specific mechanism(s) by which TYSABRI exerts its effects in multiple sclerosis and Crohn's disease have not been fully defined. | |||
In multiple sclerosis, lesions are believed to occur when activated inflammatory cells, including T-lymphocytes, cross the blood-brain barrier (BBB). Leukocyte migration across the BBB involves interaction between adhesion molecules on inflammatory cells and their counter-receptors present on endothelial cells of the vessel wall. The clinical effect of natalizumab in multiple sclerosis may be secondary to blockade of the molecular interaction of α4β1-integrin expressed by inflammatory cells with VCAM-1 on vascular endothelial cells, and with CS-1 and/or osteopontin expressed by parenchymal cells in the brain. Data from an experimental autoimmune encephalitis animal model of multiple sclerosis demonstrate reduction of leukocyte migration into brain parenchyma and reduction of plaque formation detected by magnetic resonance imaging (MRI) following repeated administration of natalizumab. The clinical significance of these animal data is unknown. | |||
In Crohn's disease, the interaction of the α4β7 integrin with the endothelial receptor MAdCAM-1 has been implicated as an important contributor to the chronic inflammation that is a hallmark of the disease. MAdCAM-1 is mainly expressed on gut endothelial cells and plays a critical role in the homing of T lymphocytes to gut lymph tissue found in Peyer's patches. MAdCAM-1 expression has been found to be increased at active sites of inflammation in patients with CD, which suggests it may play a role in the recruitment of leukocytes to the mucosa and contribute to the inflammatory response characteristic of CD. The clinical effect of natalizumab in CD may therefore be secondary to blockade of the molecular interaction of the α4ß7-integrin receptor with MAdCAM-1 expressed on the venular endothelium at inflammatory foci. VCAM-1 expression has been found to be upregulated on colonic endothelial cells in a mouse model of IBD and appears to play a role in leukocyte recruitment to sites of inflammation. The role of VCAM-1 in CD, however, is not clear. | |||
|structure=Natalizumab is a recombinant humanized IgG4ϰ monoclonal antibody produced in murine myeloma cells. Natalizumab contains human framework regions and the complementarity-determining regions of a murine antibody that binds to α4-integrin. The molecular weight of natalizumab is 149 kilodaltons | |||
|PD=Natalizumab administration increases the number of circulating leukocytes (including lymphocytes, monocytes, basophils, and eosinophils) due to inhibition of transmigration out of the vascular space. TYSABRI does not affect the absolute count of circulating neutrophils. | |||
|PK=====Multiple Sclerosis (MS) Patients==== | |||
In patients with MS, following the repeat intravenous administration of a 300 mg dose of TYSABRI, the mean ± SD maximum observed serum concentration was 110 ± 52 mcg/mL. Mean average steady-state trough concentrations ranged from 23 mcg/mL to 29 mcg/mL. The observed time to steady-state was approximately 24 weeks after every four weeks of dosing. The mean ± SD half-life, volume of distribution, and clearance of natalizumab were 11 ± 4 days, 5.7 ± 1.9 L, and 16 ± 5 mL/hour, respectively. | |||
The effects of covariates such as body weight, age, gender, and presence of anti-natalizumab antibodies on natalizumab pharmacokinetics were investigated in a population pharmacokinetic study (n=2195). Natalizumab clearance increased with body weight in a less than proportional manner such that a 43% increase in body weight resulted in a 32% increase in clearance. The presence of persistent anti-natalizumab antibodies increased natalizumab clearance approximately 3-fold. | |||
====Crohn's Disease (CD) Patients==== | |||
In patients with CD, following the repeat intravenous administration of a 300 mg dose of TYSABRI, the mean ± SD maximum observed serum concentration was 101 ± 34 mcg/mL. The mean ± SD average steady-state trough concentration was 10 ± 9 mcg/mL. The estimated time to steady-state was approximately 16 to 24 weeks after every four weeks of dosing. The mean ± SD half-life, volume of distribution, and clearance of natalizumab were 10 ± 7 days, 5.2 ± 2.8 L, and 22 ± 22 mL/hour, respectively. | |||
The effects of total body weight, age, gender, race, selected hematology and serum chemistry measures, co-administered medications (infliximab, immunosuppressants, or steroids), and the presence of anti-natalizumab antibodies were investigated in a population pharmacokinetic analysis (n=1156). The presence of anti-natalizumab antibodies was observed to increase natalizumab clearance. | |||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |||
No clastogenic or mutagenic effects of natalizumab were observed in the Ames test or in vitro chromosomal aberration assay in human lymphocytes. Natalizumab showed no effects in in vitro assays of α4-integrin positive human tumor line proliferation/cytotoxicity. Xenograft transplantation models in SCID and nude mice with two α4-integrin positive human tumor lines (leukemia, melanoma) demonstrated no increase in tumor growth rates or metastasis resulting from natalizumab treatment. | |||
Reductions in female guinea pig fertility were observed in one study at dose levels of 30 mg/kg, but not at the 10 mg/kg dose level (2.3-fold the clinical dose). A 47% reduction in pregnancy rate was observed in guinea pigs receiving 30 mg/kg relative to control. Implantations were seen in only 36% of animals having corpora lutea in the 30 mg/kg group versus 66 to 72% in the other groups. Natalizumab did not affect male fertility at doses up to 7-fold the clinical dose. | |||
====Animal Toxicology and/or Pharmacology==== | |||
In reproductive studies in monkeys and guinea pigs, there was no evidence of teratogenic effects at doses up to 30 mg/kg (7 times the human clinical dose based on a body weight comparison). In one study where female guinea pigs were exposed to natalizumab during the second half of pregnancy, a small reduction in pup survival was noted at post-natal day 14 with respect to control (3 pups/litter for the group treated with 30 mg/kg natalizumab and 4.3 pups/litter for the control group). In one of five studies that exposed monkeys or guinea pigs during pregnancy, the number of abortions in treated (30 mg/kg) monkeys was 33% versus 17% in controls. No effects on abortion rates were noted in any other study. TYSABRI underwent trans-placental transfer and produced in utero exposure in developing guinea pigs and cynomolgus monkeys. When pregnant dams were exposed to natalizumab at approximately 7-fold the clinical dose, serum levels in fetal animals at delivery were approximately 35% of maternal serum natalizumab levels. A study in pregnant cynomolgus monkeys treated at 2.3-fold the clinical dose demonstrated natalizumab-related changes in the fetus. These changes included mild anemia, reduced platelet count, increased spleen weights, and reduced liver and thymus weights associated with increased splenic extramedullary hematopoiesis, thymic atrophy, and decreased hepatic hematopoiesis. In offspring born to mothers treated with natalizumab at 7-fold the clinical dose, platelet counts were also reduced. This effect was reversed upon clearance of natalizumab. There was no evidence of anemia in these offspring. Offspring exposed in utero and via breast milk had no natalizumab-related changes in the lymphoid organs and had normal immune response to challenge with a T-cell dependent antigen. | |||
|howSupplied=* 300 mg natalizumab in 15 mL | |||
:* In a sterile single-use vial free of preservatives | |||
:* NDC 64406-008-01 | |||
|storage=* Must be refrigerated between 2 to 8°C (36° to 46°F) | |||
|packLabel=[[File:Natalizumab FDA package label.png|thumb|none|600px]] | |||
|fdaPatientInfo=====General Counseling Information==== | |||
Counsel patients to understand the risks and benefits of TYSABRI before an initial prescription is written. The patient may be educated by either the enrolled prescriber or a healthcare provider under that prescriber's direction. Instruct patients using natuzimab to: | |||
* Read the Medication Guide before starting TYSABRI and before each TYSABRI infusion. | |||
* Promptly report any new or continuously worsening symptoms that persist over several days to their prescriber. | |||
* Inform all of their physicians that they are receiving TYSABRI. | |||
* Plan to see their prescriber three months after the first infusion, six months after the first infusion, every six months thereafter, and for at least six months after discontinuing TYSABRI. | |||
====Progressive Multifocal Leukoencephalopathy==== | |||
Inform patients that Progressive Multifocal Leukoencephalopathy (PML) has occurred in patients who received TYSABRI. Instruct the patient of the importance of contacting their doctor if they develop any symptoms suggestive of PML. Instruct the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. Instruct the patient that the progression of deficits usually leads to death or severe disability over weeks or months. | |||
Instruct patients to continue to look for new signs and symptoms suggestive of PML for approximately 6 months following discontinuation of TYSABRI. | |||
====TYSABRI TOUCH Prescribing Program==== | |||
Advise the patient that TYSABRI is only available through a restricted program called the TOUCH Prescribing Program. Inform the patient of the following requirements: | |||
Patients must read the Medication Guide and sign the Patient Prescriber Enrollment Form. Advise patients that TYSABRI is available only from certified pharmacies and infusion centers participating in the program. | |||
====Herpes Encephalitis/Meningitis==== | |||
Inform patients that TYSABRI increases the risk of developing encephalitis and meningitis caused by herpes simplex and varicella zoster viruses. Instruct patients to report immediately if they experience symptoms such as fever, headache and confusion. | |||
====Hepatotoxicity==== | |||
Inform patients that TYSABRI may cause liver injury. Instruct patients treated with Tysabri to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. | |||
====Hypersensitivity Reactions==== | |||
Instruct patients to report immediately if they experience symptoms consistent with a hypersensitivity reaction (e.g., urticaria with or without associated symptoms) during or following an infusion of TYSABRI. | |||
====Immunosuppression/Infections==== | |||
Inform patients that TYSABRI may lower the ability of their immune system to fight infections. Instruct the patient of the importance of contacting their doctor if they develop any symptoms of infection. | |||
|alcohol=Alcohol-Natalizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
{{LabelImage | |||
|fileName=Natalizumab 20mg.png | |||
}} | |||
{{drugbox-mab | | {{drugbox-mab | | ||
| image = | | image = | ||
Revision as of 21:11, 14 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete Boxed Warning.
Natalizumab increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability. Risk factors for the development of PML include duration of therapy, prior use of immunosuppressants, and presence of anti-JCV antibodies. These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab.
Healthcare professionals should monitor patients on natalizumab for any new sign or symptom that may be suggestive of PML. TYSABRI dosing should be withheld immediately at the first sign or symptom suggestive of PML. For diagnosis, an evaluation that includes a gadolinium-enhanced magnetic resonance imaging (MRI) scan of the brain and, when indicated, cerebrospinal fluid analysis for JC viral DNA are recommended. Because of the risk of PML, natalizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the TOUCH Prescribing Program. |
Overview
Natalizumab is a monoclonal antibody that is FDA approved for the treatment of multiple sclerosis (MS) and Crohn's disease (CD). There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, fatigue, arthralgia, urinary tract infection, lower respiratory tract infection, gastroenteritis, vaginitis, depression, pain in extremity, abdominal discomfort, diarrhea NOS, and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Multiple Sclerosis (MS)
- Is indicated as monotherapy for the treatment of patients with relapsing forms of multiple sclerosis.
- Natalizumab increases the risk of PML.
- When initiating and continuing treatment with natalizumab, physicians should consider whether the expected benefit of natalizumab is sufficient to offset this risk.
- Dosage:
- 300 mg intravenous infusion over one hour every four weeks.
Crohn's Disease (CD)
- Is indicated for inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn's disease with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional CD therapies and inhibitors of TNF-α.
- Natalizumab should not be used in combination with immunosuppressants (e.g., 6-mercaptopurine, azathioprine, cyclosporine, or methotrexate) or inhibitors of TNF-α.
- Dosage:
- 300 mg intravenous infusion over one hour every four weeks
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Natalizumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Natalizumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Natalizumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Natalizumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Natalizumab in pediatric patients.
Contraindications
- TYSABRI is contraindicated in patients who have or have had progressive multifocal leukoencephalopathy (PML).
- TYSABRI should not be administered to a patient who has had a hypersensitivity reaction to TYSABRI. Observed reactions range from urticaria to anaphylaxis
Warnings
|
WARNING: PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete Boxed Warning.
Natalizumab increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability. Risk factors for the development of PML include duration of therapy, prior use of immunosuppressants, and presence of anti-JCV antibodies. These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab.
Healthcare professionals should monitor patients on natalizumab for any new sign or symptom that may be suggestive of PML. TYSABRI dosing should be withheld immediately at the first sign or symptom suggestive of PML. For diagnosis, an evaluation that includes a gadolinium-enhanced magnetic resonance imaging (MRI) scan of the brain and, when indicated, cerebrospinal fluid analysis for JC viral DNA are recommended. Because of the risk of PML, natalizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the TOUCH Prescribing Program. |
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability, has occurred in patients who have received TYSABRI.
Three factors that are known to increase the risk of PML in TYSABRI-treated patients have been identified:
- Longer treatment duration, especially beyond 2 years. There is limited experience in patients who have received more than 6 years of TYSABRI treatment.
- Prior treatment with an immunosuppressant (e.g., mitoxantrone, azathioprine, methotrexate, cyclophosphamide, mycophenolate mofetil).
- The presence of anti-JCV antibodies. Patients who are anti-JCV antibody positive have a higher risk for developing PML.
These factors should be considered in the context of expected benefit when initiating and continuing treatment with TYSABRI.
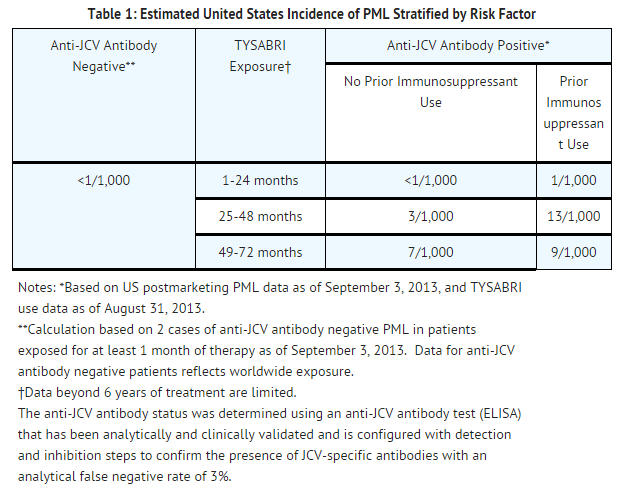
Infection by the JC virus is required for the development of PML. Anti-JCV antibody testing should not be used to diagnose PML. Anti-JCV antibody negative status indicates that exposure to the JC virus has not been detected. Patients who are anti-JCV antibody negative have a lower risk of PML than those who are positive. Patients who are anti-JCV antibody negative are still at risk for the development of PML due to the potential for a new JCV infection or a false negative test result. The reported rate of seroconversion in patients with MS (changing from anti-JCV antibody negative to positive and remaining positive in subsequent testing) is 3 to 8 percent annually. In addition, some patients' serostatus may change intermittently. Therefore, patients with a negative anti-JCV antibody test result should be retested periodically. For purposes of risk assessment, a patient with a positive anti-JCV antibody test at any time is considered anti-JCV antibody positive regardless of the results of any prior or subsequent anti-JCV antibody testing. When assessed, anti-JCV antibody status should be determined using an analytically and clinically validated immunoassay. Anti-JCV antibody testing should not be performed for at least two weeks following plasma exchange due to the removal of antibodies from the serum.
There are no known interventions that can reliably prevent PML or adequately treat PML if it occurs. It is not known whether early detection of PML and discontinuation of TYSABRI will mitigate the disease. PML has been reported following discontinuation of TYSABRI in patients who did not have findings suggestive of PML at the time of discontinuation. Patients should continue to be monitored for any new signs or symptoms that may be suggestive of PML for at least six months following discontinuation of TYSABRI.
Ordinarily, patients receiving chronic immunosuppressant or immunomodulatory therapy or who have systemic medical conditions resulting in significantly compromised immune system function should not be treated with TYSABRI.
Because of the risk of PML, TYSABRI is available only under a restricted distribution program, the TOUCH® Prescribing Program.
In multiple sclerosis patients, an MRI scan should be obtained prior to initiating therapy with TYSABRI. This MRI may be helpful in differentiating subsequent multiple sclerosis symptoms from PML.
In Crohn's disease patients, a baseline brain MRI may also be helpful to distinguish pre-existent lesions from newly developed lesions, but brain lesions at baseline that could cause diagnostic difficulty while on TYSABRI therapy are uncommon.
Healthcare professionals should monitor patients on TYSABRI for any new sign or symptom suggestive of PML. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. The progression of deficits usually leads to death or severe disability over weeks or months. Withhold TYSABRI dosing immediately at the first sign or symptom suggestive of PML.
For diagnosis of PML, an evaluation including a gadolinium-enhanced MRI scan of the brain and, when indicated, cerebrospinal fluid analysis for JC viral DNA are recommended. If the initial evaluations for PML are negative but clinical suspicion for PML remains, continue to withhold TYSABRI dosing and repeat the evaluations.
There are no known interventions that can adequately treat PML if it occurs. Three sessions of plasma exchange over 5 to 8 days were shown to accelerate TYSABRI clearance in a study of 12 patients with MS who did not have PML, although in the majority of patients alpha-4 integrin receptor binding remained high. Adverse events which may occur during plasma exchange include clearance of other medications and volume shifts, which have the potential to lead to hypotension or pulmonary edema. Although plasma exchange has not been studied in TYSABRI treated patients with PML, it has been used in such patients in the postmarketing setting to remove TYSABRI more quickly from the circulation. Anti-JCV antibody testing should not be performed during or for at least two weeks following plasma exchange due to the removal of antibodies from the serum.
Immune reconstitution inflammatory syndrome (IRIS) has been reported in the majority of TYSABRI treated patients who developed PML and subsequently discontinued TYSABRI. In almost all cases, IRIS occurred after plasma exchange was used to eliminate circulating TYSABRI. It presents as a clinical decline in the patient's condition after TYSABRI removal (and in some cases after apparent clinical improvement) that may be rapid, can lead to serious neurological complications or death and is often associated with characteristic changes in the MRI. TYSABRI has not been associated with IRIS in patients discontinuing treatment with TYSABRI for reasons unrelated to PML. In TYSABRI-treated patients with PML, IRIS has been reported within days to several weeks after plasma exchange. Monitoring for development of IRIS and appropriate treatment of the associated inflammation should be undertaken.
TYSABRI TOUCH Prescribing Program
TYSABRI is available only through a restricted program under a REMS called the TOUCH® Prescribing Program because of the risk of PML.
For prescribers and patients, the TOUCH® Prescribing Program has two components: MS TOUCH® (for patients with multiple sclerosis) and CD TOUCH® (for patients with Crohn's disease).
Selected requirements of the TOUCH® Prescribing Program include the following:
- Prescribers must be certified and comply with the following:
- Review the TOUCH Prescribing Program prescriber educational materials, including the full prescribing information.
- Educate patients on the benefits and risks of treatment with TYSABRI, ensure that patients receive the Medication Guide, and encourage them to ask questions.
- Review, complete, and sign the Patient-Prescriber Enrollment Form.
- Evaluate patients three months after the first infusion, six months after the first infusion, every six months thereafter, and for at least six months after discontinuing TYSABRI.
- Determine every six months whether patients should continue on treatment and, if so, authorize treatment for another six months.
- Submit to Biogen Idec the “TYSABRI Patient Status Report and Reauthorization Questionnaire” six months after initiating treatment and every six months thereafter.
- Complete an “Initial Discontinuation Questionnaire” when TYSABRI is discontinued and a “6-Month Discontinuation Questionnaire” following discontinuation of TYSABRI.
- Report cases of PML, hospitalizations due to opportunistic infections, or deaths to Biogen Idec at 1-800-456-2255 as soon as possible.
- Patients must be enrolled in the TOUCH Prescribing Program, read the Medication Guide, understand the risks associated with TYSABRI and complete and sign the Patient-Prescriber Enrollment Form.
- Pharmacies and infusion centers must be specially certified to dispense or infuse TYSABRI.
Herpes Encephalitis and Meningitis
Natalizumab increases the risk of developing encephalitis and meningitis caused by herpes simplex and varicella zoster viruses. Serious, life-threatening, and sometimes fatal cases have been reported in the postmarketing setting in multiple sclerosis patients receiving TYSABRI. Laboratory confirmation in those cases was based on positive PCR for viral DNA in the cerebrospinal fluid. The duration of treatment with TYSABRI prior to onset ranged from a few months to several years. Monitor patients receiving TYSABRI for signs and symptoms of meningitis and encephalitis. If herpes encephalitis or meningitis occurs, TYSBARI should be discontinued, and appropriate treatment for herpes encephalitis/meningitis should be administered.
Hepatotoxicity
Clinically significant liver injury, including acute liver failure requiring transplant, has been reported in patients treated with TYSABRI in the postmarketing setting. Signs of liver injury, including markedly elevated serum hepatic enzymes and elevated total bilirubin, occurred as early as six days after the first dose; signs of liver injury have also been reported for the first time after multiple doses. In some patients, liver injury recurred upon rechallenge, providing evidence that TYSABRI caused the injury. The combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury that may lead to death or the need for a liver transplant in some patients.
Natalizumab should be discontinued in patients with jaundice or other evidence of significant liver injury (e.g., laboratory evidence).
Hypersensitivity/Antibody Formation
Hypersensitivity reactions have occurred in patients receiving TYSABRI, including serious systemic reactions (e.g., anaphylaxis) which occurred at an incidence of <1%. These reactions usually occur within two hours of the start of the infusion. Symptoms associated with these reactions can include urticaria, dizziness, fever, rash, rigors, pruritus, nausea, flushing, hypotension, dyspnea, and chest pain. Generally, these reactions are associated with antibodies to TYSABRI.
If a hypersensitivity reaction occurs, discontinue administration of TYSABRI and initiate appropriate therapy. Patients who experience a hypersensitivity reaction should not be re-treated with TYSABRI. Hypersensitivity reactions were more frequent in patients with antibodies to TYSABRI compared to patients who did not develop antibodies to TYSABRI in both MS and CD studies. Therefore, the possibility of antibodies to TYSABRI should be considered in patients who have hypersensitivity reactions.
Antibody testing: If the presence of persistent antibodies is suspected, antibody testing should be performed. Antibodies may be detected and confirmed with sequential serum antibody tests. Antibodies detected early in the treatment course (e.g., within the first six months) may be transient and disappear with continued dosing. Repeat testing at three months after the initial positive result is recommended in patients in whom antibodies are detected to confirm that antibodies are persistent. Prescribers should consider the overall benefits and risks of TYSABRI in a patient with persistent antibodies.
Experience with monoclonal antibodies, including TYSABRI, suggests that patients who receive therapeutic monoclonal antibodies after an extended period without treatment may be at higher risk of hypersensitivity reactions than patients who received regularly scheduled treatment. Given that patients with persistent antibodies to TYSABRI experience reduced efficacy, and that hypersensitivity reactions are more common in such patients, consideration should be given to testing for the presence of antibodies in patients who wish to recommence therapy following a dose interruption. Following a period of dose interruption, patients testing negative for antibodies prior to re-dosing have a risk of antibody development with re-treatment that is similar to TYSABRI naïve patients.
Immunosuppression/Infections
The immune system effects of TYSABRI may increase the risk for infections. In Study MS1, certain types of infections, including pneumonias and urinary tract infections (including serious cases), gastroenteritis, vaginal infections, tooth infections, tonsillitis, and herpes infections, occurred more often in TYSABRI-treated patients than in placebo-treated patients. One opportunistic infection, a cryptosporidial gastroenteritis with a prolonged course, was observed in a patient who received TYSABRI in Study MS1.
In Studies MS1 and MS2, an increase in infections was seen in patients concurrently receiving short courses of corticosteroids. However, the increase in infections in TYSABRI-treated patients who received steroids was similar to the increase in placebo-treated patients who received steroids.
In CD clinical studies, opportunistic infections (pneumocystis carinii pneumonia, pulmonary mycobacterium avium intracellulare, bronchopulmonary aspergillosis, and burkholderia cepacia) have been observed in <1% of TYSABRI-treated patients; some of these patients were receiving concurrent immunosuppressants.
In Studies CD1 and CD2, an increase in infections was seen in patients concurrently receiving corticosteroids. However, the increase in infections was similar in placebo-treated and TYSABRI-treated patients who received steroids.
Concurrent use of antineoplastic, immunosuppressant, or immunomodulating agents may further increase the risk of infections, including PML and other opportunistic infections, over the risk observed with use of TYSABRI alone. The safety and efficacy of TYSABRI in combination with antineoplastic, immunosuppressant, or immunomodulating agents have not been established. Patients receiving chronic immunosuppressant or immunomodulatory therapy or who have systemic medical conditions resulting in significantly compromised immune system function should not ordinarily be treated with TYSABRI. The risk of PML is also increased in patients who have been treated with an immunosuppressant prior to receiving TYSABRI.
For patients with Crohn's disease who start TYSABRI while on chronic corticosteroids, commence steroid withdrawal as soon as a therapeutic benefit has occurred. If the patient cannot discontinue systemic corticosteroids within six months, discontinue TYSABRI.
Laboratory Test Abnormalities
In clinical trials, TYSABRI was observed to induce increases in circulating lymphocytes, monocytes, eosinophils, basophils, and nucleated red blood cells. Observed changes persisted during TYSABRI exposure, but were reversible, returning to baseline levels usually within 16 weeks after the last dose. Elevations of neutrophils were not observed. TYSABRI induces mild decreases in hemoglobin levels that are frequently transient.
Immunizations
No data are available on the effects of vaccination in patients receiving TYSABRI. No data are available on the secondary transmission of infection by live vaccines in patients receiving TYSABRI.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions (incidence ≥ 10%) were headache and fatigue in both the multiple sclerosis (MS) and Crohn's disease (CD) studies. Other common adverse reactions (incidence ≥ 10%) in the MS population were arthralgia, urinary tract infection, lower respiratory tract infection, gastroenteritis, vaginitis, depression, pain in extremity, abdominal discomfort, diarrhea NOS, and rash. Other common adverse reactions (incidence ≥ 10%) in the CD population were upper respiratory tract infections and nausea.
The most frequently reported adverse reactions resulting in clinical intervention (i.e., discontinuation of TYSABRI), in the MS studies were urticaria (1%) and other hypersensitivity reactions (1%), and in the CD studies (Studies CD1 and CD2) were the exacerbation of Crohn's disease (4.2%) and acute hypersensitivity reactions (1.5%).
A total of 1617 multiple sclerosis patients in controlled studies received TYSABRI, with a median duration of exposure of 28 months. A total of 1563 patients received TYSABRI in all CD studies for a median exposure of 5 months; of these patients, 33% (n=518) received at least one year of treatment and 19% (n=297) received at least two years of treatment.
Multiple Sclerosis Clinical Studies
The most frequently reported serious adverse reactions in Study MS1 [see Clinical Studies (14.1)] with TYSABRI were infections (3.2% versus 2.6% in placebo, including urinary tract infection [0.8% versus 0.3%] and pneumonia [0.6% versus 0%]), acute hypersensitivity reactions (1.1% versus 0.3%, including anaphylaxis/anaphylactoid reaction [0.8% versus 0%]), depression (1.0% versus 1.0%, including suicidal ideation or attempt [0.6% versus 0.3%]), and cholelithiasis (1.0% versus 0.3%). In Study MS2, serious adverse reactions of appendicitis were also more common in patients who received TYSABRI (0.8% versus 0.2% in placebo).
TABLE 2 enumerates adverse reactions and selected laboratory abnormalities that occurred in Study MS1 at an incidence of at least 1 percentage point higher in TYSABRI-treated patients than was observed in placebo-treated patients.
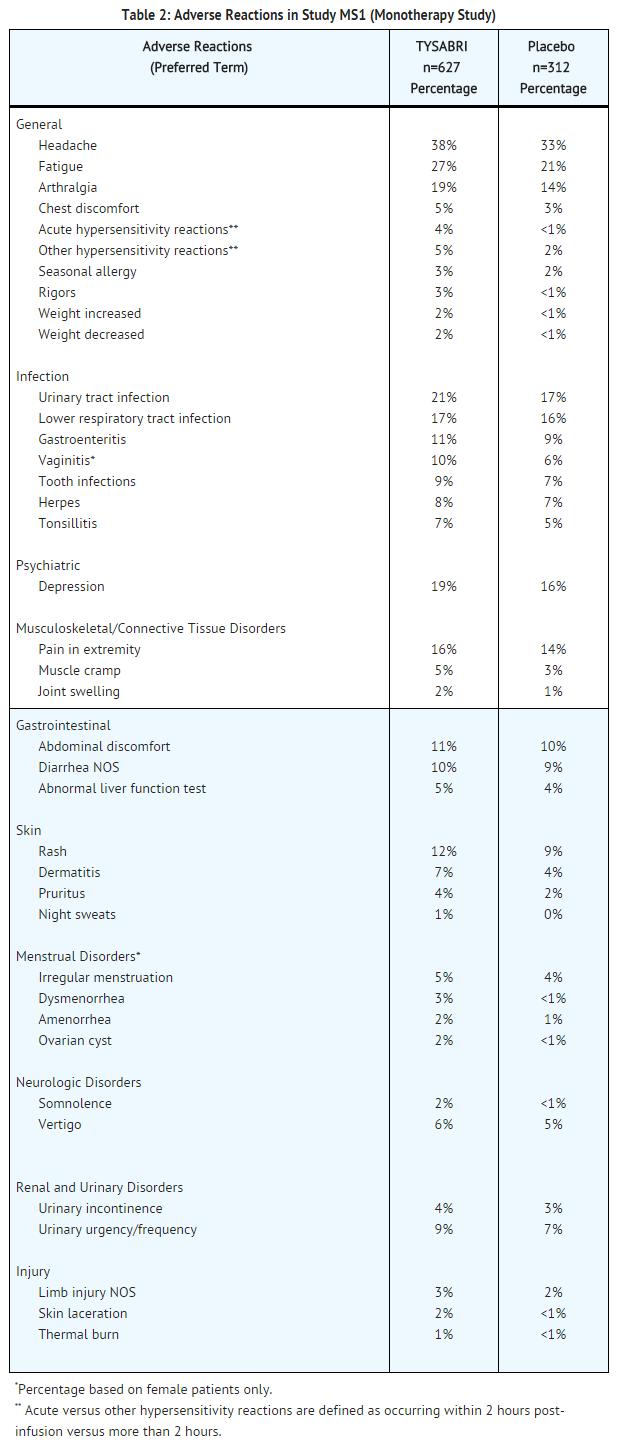
In Study MS2, peripheral edema was more common in patients who received TYSABRI (5% versus 1% in placebo).
Crohn's Disease Clinical Studies
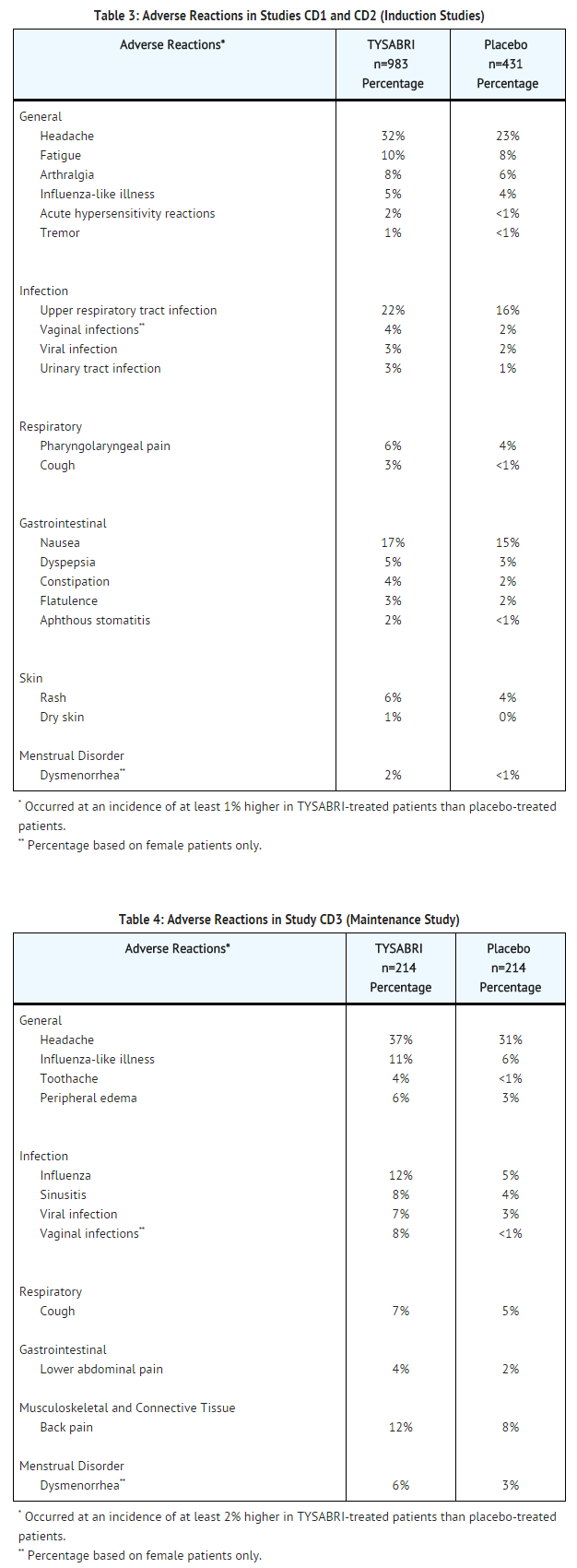
The following serious adverse events in the induction Studies CD1 and CD2 were reported more commonly with TYSABRI than placebo and occurred at an incidence of at least 0.3%: intestinal obstruction or stenosis (2% vs. 1% in placebo), acute hypersensitivity reactions (0.5% vs. 0%), abdominal adhesions (0.3% vs. 0%), and cholelithiasis (0.3% vs. 0%). Similar serious adverse events were seen in the maintenance Study CD3. TABLE 3 enumerates adverse drug reactions that occurred in Studies CD1 and CD2 (median exposure of 2.8 months). TABLE 4 enumerates adverse drug reactions that occurred in Study CD3 (median exposure of 11.0 months).
Infections
Progressive Multifocal Leukoencephalopathy (PML) occurred in three patients who received TYSABRI in clinical trials. Two cases of PML were observed in the 1869 patients with multiple sclerosis who were treated for a median of 120 weeks. These two patients had received TYSABRI in addition to interferon beta-1a. The third case occurred after eight doses in one of the 1043 patients with Crohn's disease who were evaluated for PML. In the postmarketing setting, additional cases of PML have been reported in TYSABRI-treated multiple sclerosis and Crohn's disease patients who were not receiving concomitant immunomodulatory therapy.
In Studies MS1 and MS2, the rate of any type of infection was approximately 1.5 per patient-year in both TYSABRI-treated patients and placebo-treated patients. The infections were predominately upper respiratory tract infections, influenza, and urinary tract infections. In Study MS1, the incidence of serious infection was approximately 3% in TYSABRI-treated patients and placebo-treated patients. Most patients did not interrupt treatment with TYSABRI during infections. The only opportunistic infection in the multiple sclerosis clinical trials was a case of cryptosporidial gastroenteritis with a prolonged course.
In Studies CD1 and CD2, the rate of any type of infection was 1.7 per patient-year in TYSABRI-treated patients and 1.4 per patient-year in placebo-treated patients. In Study CD3, the incidence of any type of infection was 1.7 per patient-year in TYSABRI-treated patients and was similar in placebo-treated patients. The most common infections were nasopharyngitis, upper respiratory tract infection, and influenza. The majority of patients did not interrupt TYSABRI therapy during infections and recovery occurred with appropriate treatment. Concurrent use of TYSABRI in CD clinical trials with chronic steroids and/or methotrexate, 6-MP, and azathioprine did not result in an increase in overall infections compared to TYSABRI alone; however, the concomitant use of such agents could lead to an increased risk of serious infections.
In Studies CD1 and CD2, the incidence of serious infection was approximately 2.1% in both TYSABRI-treated patients and placebo-treated patients. In Study CD3, the incidence of serious infection was approximately 3.3% in TYSABRI-treated patients and approximately 2.8% in placebo-treated patients.
In clinical studies for CD, opportunistic infections (pneumocystis carinii pneumonia, pulmonary mycobacterium avium intracellulare, bronchopulmonary aspergillosis, and burkholderia cepacia) have been observed in <1% of TYSABRI-treated patients; some of these patients were receiving concurrent immunosuppressants. Two serious non-bacterial meningitides occurred in TYSABRI-treated patients compared to none in placebo-treated patients.
An infusion-related reaction was defined in clinical trials as any adverse event occurring within two hours of the start of an infusion. In MS clinical trials, approximately 24% of TYSABRI-treated multiple sclerosis patients experienced an infusion-related reaction, compared to 18% of placebo-treated patients. In the controlled CD clinical trials, infusion-related reactions occurred in approximately 11% of patients treated with TYSABRI compared to 7% of placebo-treated patients. Reactions more common in the TYSABRI-treated MS patients compared to the placebo-treated MS patients included headache, dizziness, fatigue, urticaria, pruritus, and rigors. Acute urticaria was observed in approximately 2% of patients. Other hypersensitivity reactions were observed in 1% of patients receiving TYSABRI. Serious systemic hypersensitivity infusion reactions occurred in <1% of patients. All patients recovered with treatment and/or discontinuation of the infusion.
Infusion-related reactions more common in CD patients receiving TYSABRI than those receiving placebo included headache, nausea, urticaria, pruritus, and flushing. Serious infusion reactions occurred in Studies CD1, CD2, and CD3 at an incidence of <1% in TYSABRI-treated patients.
MS and CD patients who became persistently positive for antibodies to TYSABRI were more likely to have an infusion-related reaction than those who were antibody-negative.
Postmarketing Experience
There is limited information regarding Natalizumab Postmarketing Experience in the drug label.
Drug Interactions
Because of the potential for increased risk of PML and other infections, Crohn's disease patients receiving TYSABRI should not be treated with concomitant immunosuppressants (e.g., 6-mercaptopurine, azathioprine, cyclosporine, or methotrexate) or inhibitors of TNF-α, and corticosteroids should be tapered in those patients with Crohn's disease who are on chronic corticosteroids when they start TYSABRI therapy. Ordinarily, MS patients receiving chronic immunosuppressant or immunomodulatory therapy should not be treated with TYSABRI.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Natalizumab has been shown to reduce pup survival in guinea pigs when given in doses 7 times the human dose, and has been shown to have hematologic effects on the fetus in monkeys when given in doses 2.3 times the human dose. There are no adequate and well-controlled studies in pregnant women. TYSABRI should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS): C
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Natalizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Natalizumab during labor and delivery.
Nursing Mothers
Natalizumab has been detected in human milk. The effects of this exposure on infants are unknown.
Pediatric Use
Safety and effectiveness of TYSABRI in pediatric patients with multiple sclerosis or Crohn's disease below the age of 18 years have not been established. TYSABRI is not indicated for use in pediatric patients.
Geriatic Use
Clinical studies of TYSABRI did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Natalizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Natalizumab with respect to specific racial populations.
Renal Impairment
Pharmacokinetics of natalizumab in patients with renal or hepatic insufficiency have not been studied.
Hepatic Impairment
Pharmacokinetics of natalizumab in patients with renal or hepatic insufficiency have not been studied.
Females of Reproductive Potential and Males
Natalizumab did not affect male fertility at doses up to 7-fold the clinical dose.
Immunocompromised Patients
There is no FDA guidance one the use of Natalizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Natalizumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Natalizumab and IV administrations.
Overdosage
Safety of doses higher than 300 mg has not been adequately evaluated. The maximum amount of TYSABRI that can be safely administered has not been determined.
Pharmacology
Natalizumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | alpha-4 integrin |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | 149 kDa |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | ? |
| Half life | 11 ± 4 days |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Intravenous infusion |
Mechanism of Action
Natalizumab binds to the α4-subunit of α4β1 and α4β7 integrins expressed on the surface of all leukocytes except neutrophils, and inhibits the α4-mediated adhesion of leukocytes to their counter-receptor(s). The receptors for the α4 family of integrins include vascular cell adhesion molecule-1 (VCAM-1), which is expressed on activated vascular endothelium, and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) present on vascular endothelial cells of the gastrointestinal tract. Disruption of these molecular interactions prevents transmigration of leukocytes across the endothelium into inflamed parenchymal tissue. In vitro, anti-α4-integrin antibodies also block α4-mediated cell binding to ligands such as osteopontin and an alternatively spliced domain of fibronectin, connecting segment-1 (CS-1). In vivo, natalizumab may further act to inhibit the interaction of α4-expressing leukocytes with their ligand(s) in the extracellular matrix and on parenchymal cells, thereby inhibiting further recruitment and inflammatory activity of activated immune cells.
The specific mechanism(s) by which TYSABRI exerts its effects in multiple sclerosis and Crohn's disease have not been fully defined.
In multiple sclerosis, lesions are believed to occur when activated inflammatory cells, including T-lymphocytes, cross the blood-brain barrier (BBB). Leukocyte migration across the BBB involves interaction between adhesion molecules on inflammatory cells and their counter-receptors present on endothelial cells of the vessel wall. The clinical effect of natalizumab in multiple sclerosis may be secondary to blockade of the molecular interaction of α4β1-integrin expressed by inflammatory cells with VCAM-1 on vascular endothelial cells, and with CS-1 and/or osteopontin expressed by parenchymal cells in the brain. Data from an experimental autoimmune encephalitis animal model of multiple sclerosis demonstrate reduction of leukocyte migration into brain parenchyma and reduction of plaque formation detected by magnetic resonance imaging (MRI) following repeated administration of natalizumab. The clinical significance of these animal data is unknown.
In Crohn's disease, the interaction of the α4β7 integrin with the endothelial receptor MAdCAM-1 has been implicated as an important contributor to the chronic inflammation that is a hallmark of the disease. MAdCAM-1 is mainly expressed on gut endothelial cells and plays a critical role in the homing of T lymphocytes to gut lymph tissue found in Peyer's patches. MAdCAM-1 expression has been found to be increased at active sites of inflammation in patients with CD, which suggests it may play a role in the recruitment of leukocytes to the mucosa and contribute to the inflammatory response characteristic of CD. The clinical effect of natalizumab in CD may therefore be secondary to blockade of the molecular interaction of the α4ß7-integrin receptor with MAdCAM-1 expressed on the venular endothelium at inflammatory foci. VCAM-1 expression has been found to be upregulated on colonic endothelial cells in a mouse model of IBD and appears to play a role in leukocyte recruitment to sites of inflammation. The role of VCAM-1 in CD, however, is not clear.
Structure
Natalizumab is a recombinant humanized IgG4ϰ monoclonal antibody produced in murine myeloma cells. Natalizumab contains human framework regions and the complementarity-determining regions of a murine antibody that binds to α4-integrin. The molecular weight of natalizumab is 149 kilodaltons
Pharmacodynamics
Natalizumab administration increases the number of circulating leukocytes (including lymphocytes, monocytes, basophils, and eosinophils) due to inhibition of transmigration out of the vascular space. TYSABRI does not affect the absolute count of circulating neutrophils.
Pharmacokinetics
Multiple Sclerosis (MS) Patients
In patients with MS, following the repeat intravenous administration of a 300 mg dose of TYSABRI, the mean ± SD maximum observed serum concentration was 110 ± 52 mcg/mL. Mean average steady-state trough concentrations ranged from 23 mcg/mL to 29 mcg/mL. The observed time to steady-state was approximately 24 weeks after every four weeks of dosing. The mean ± SD half-life, volume of distribution, and clearance of natalizumab were 11 ± 4 days, 5.7 ± 1.9 L, and 16 ± 5 mL/hour, respectively.
The effects of covariates such as body weight, age, gender, and presence of anti-natalizumab antibodies on natalizumab pharmacokinetics were investigated in a population pharmacokinetic study (n=2195). Natalizumab clearance increased with body weight in a less than proportional manner such that a 43% increase in body weight resulted in a 32% increase in clearance. The presence of persistent anti-natalizumab antibodies increased natalizumab clearance approximately 3-fold.
Crohn's Disease (CD) Patients
In patients with CD, following the repeat intravenous administration of a 300 mg dose of TYSABRI, the mean ± SD maximum observed serum concentration was 101 ± 34 mcg/mL. The mean ± SD average steady-state trough concentration was 10 ± 9 mcg/mL. The estimated time to steady-state was approximately 16 to 24 weeks after every four weeks of dosing. The mean ± SD half-life, volume of distribution, and clearance of natalizumab were 10 ± 7 days, 5.2 ± 2.8 L, and 22 ± 22 mL/hour, respectively.
The effects of total body weight, age, gender, race, selected hematology and serum chemistry measures, co-administered medications (infliximab, immunosuppressants, or steroids), and the presence of anti-natalizumab antibodies were investigated in a population pharmacokinetic analysis (n=1156). The presence of anti-natalizumab antibodies was observed to increase natalizumab clearance.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No clastogenic or mutagenic effects of natalizumab were observed in the Ames test or in vitro chromosomal aberration assay in human lymphocytes. Natalizumab showed no effects in in vitro assays of α4-integrin positive human tumor line proliferation/cytotoxicity. Xenograft transplantation models in SCID and nude mice with two α4-integrin positive human tumor lines (leukemia, melanoma) demonstrated no increase in tumor growth rates or metastasis resulting from natalizumab treatment.
Reductions in female guinea pig fertility were observed in one study at dose levels of 30 mg/kg, but not at the 10 mg/kg dose level (2.3-fold the clinical dose). A 47% reduction in pregnancy rate was observed in guinea pigs receiving 30 mg/kg relative to control. Implantations were seen in only 36% of animals having corpora lutea in the 30 mg/kg group versus 66 to 72% in the other groups. Natalizumab did not affect male fertility at doses up to 7-fold the clinical dose.
Animal Toxicology and/or Pharmacology
In reproductive studies in monkeys and guinea pigs, there was no evidence of teratogenic effects at doses up to 30 mg/kg (7 times the human clinical dose based on a body weight comparison). In one study where female guinea pigs were exposed to natalizumab during the second half of pregnancy, a small reduction in pup survival was noted at post-natal day 14 with respect to control (3 pups/litter for the group treated with 30 mg/kg natalizumab and 4.3 pups/litter for the control group). In one of five studies that exposed monkeys or guinea pigs during pregnancy, the number of abortions in treated (30 mg/kg) monkeys was 33% versus 17% in controls. No effects on abortion rates were noted in any other study. TYSABRI underwent trans-placental transfer and produced in utero exposure in developing guinea pigs and cynomolgus monkeys. When pregnant dams were exposed to natalizumab at approximately 7-fold the clinical dose, serum levels in fetal animals at delivery were approximately 35% of maternal serum natalizumab levels. A study in pregnant cynomolgus monkeys treated at 2.3-fold the clinical dose demonstrated natalizumab-related changes in the fetus. These changes included mild anemia, reduced platelet count, increased spleen weights, and reduced liver and thymus weights associated with increased splenic extramedullary hematopoiesis, thymic atrophy, and decreased hepatic hematopoiesis. In offspring born to mothers treated with natalizumab at 7-fold the clinical dose, platelet counts were also reduced. This effect was reversed upon clearance of natalizumab. There was no evidence of anemia in these offspring. Offspring exposed in utero and via breast milk had no natalizumab-related changes in the lymphoid organs and had normal immune response to challenge with a T-cell dependent antigen.
Clinical Studies
There is limited information regarding Natalizumab Clinical Studies in the drug label.
How Supplied
- 300 mg natalizumab in 15 mL
- In a sterile single-use vial free of preservatives
- NDC 64406-008-01
Storage
- Must be refrigerated between 2 to 8°C (36° to 46°F)
Images
Drug Images
{{#ask: Page Name::Natalizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
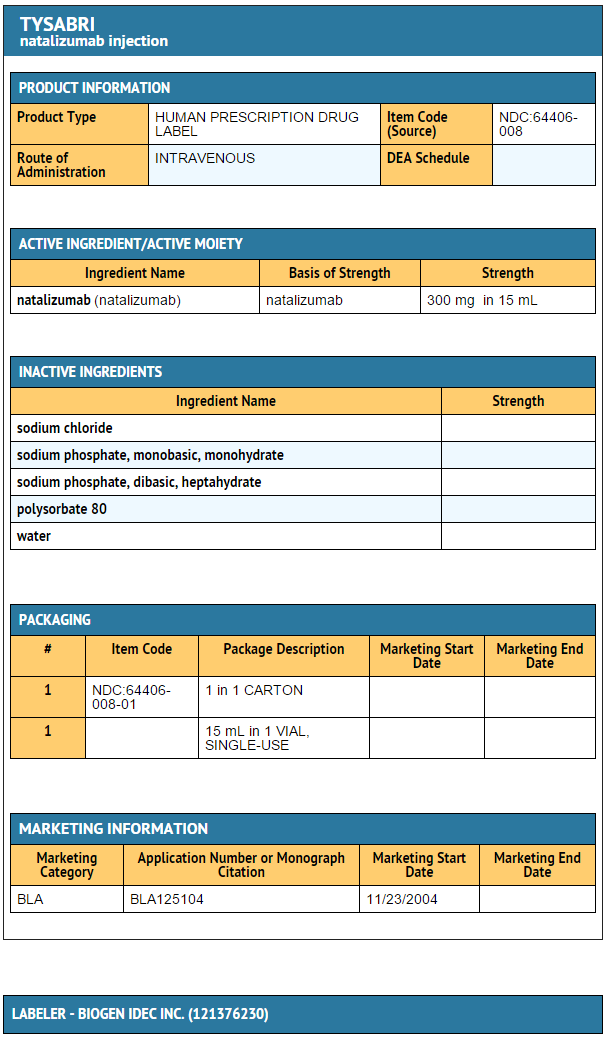
{{#ask: Label Page::Natalizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
General Counseling Information
Counsel patients to understand the risks and benefits of TYSABRI before an initial prescription is written. The patient may be educated by either the enrolled prescriber or a healthcare provider under that prescriber's direction. Instruct patients using natuzimab to:
- Read the Medication Guide before starting TYSABRI and before each TYSABRI infusion.
- Promptly report any new or continuously worsening symptoms that persist over several days to their prescriber.
- Inform all of their physicians that they are receiving TYSABRI.
- Plan to see their prescriber three months after the first infusion, six months after the first infusion, every six months thereafter, and for at least six months after discontinuing TYSABRI.
Progressive Multifocal Leukoencephalopathy
Inform patients that Progressive Multifocal Leukoencephalopathy (PML) has occurred in patients who received TYSABRI. Instruct the patient of the importance of contacting their doctor if they develop any symptoms suggestive of PML. Instruct the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. Instruct the patient that the progression of deficits usually leads to death or severe disability over weeks or months.
Instruct patients to continue to look for new signs and symptoms suggestive of PML for approximately 6 months following discontinuation of TYSABRI.
TYSABRI TOUCH Prescribing Program
Advise the patient that TYSABRI is only available through a restricted program called the TOUCH Prescribing Program. Inform the patient of the following requirements:
Patients must read the Medication Guide and sign the Patient Prescriber Enrollment Form. Advise patients that TYSABRI is available only from certified pharmacies and infusion centers participating in the program.
Herpes Encephalitis/Meningitis
Inform patients that TYSABRI increases the risk of developing encephalitis and meningitis caused by herpes simplex and varicella zoster viruses. Instruct patients to report immediately if they experience symptoms such as fever, headache and confusion.
Hepatotoxicity
Inform patients that TYSABRI may cause liver injury. Instruct patients treated with Tysabri to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
Hypersensitivity Reactions
Instruct patients to report immediately if they experience symptoms consistent with a hypersensitivity reaction (e.g., urticaria with or without associated symptoms) during or following an infusion of TYSABRI.
Immunosuppression/Infections
Inform patients that TYSABRI may lower the ability of their immune system to fight infections. Instruct the patient of the importance of contacting their doctor if they develop any symptoms of infection.
Precautions with Alcohol
Alcohol-Natalizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Natalizumab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Natalizumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Natalizumab |Label Name=Natalizumab 20mg.png
}}
Template:Drugbox-mab Natalizumab is a prescription drug co-marketed by Biogen Idec and Élan as Tysabri. It was previously named Antegren. Natalizumab is administered by infusion and has proven effective in the treatment of multiple sclerosis and Crohn's disease. Three months after its approval by the FDA, the drug was voluntarily withdrawn after being linked to adverse events including one death. After an intensive safety evaluation, the drug was re-approved by the FDA as a potentially significant advancement over existing therapies and returned to the marketplace under a program of restricted distribution and regular patient evaluations.
Description
Natalizumab is a humanized monoclonal antibody against integrin-α4 that has proven efficacy in the treatment of two serious autoimmune disorders: multiple sclerosis (MS) and Crohn's disease (CD).
In MS, natalizumab was shown to reduce relapses by 67% vs. a placebo. It slowed the progression of disability (as measured by EDSS) by 42%. While it is impossible to compare results across different clinical trials, the older generation drugs, interferons and glatiramer acetate (Copaxone), are generally acknowledged as demonstrating about a 30-35% decrease in relapse rate vs. placebo; and only two drugs have been shown to decrease the progression of disability, but again only by around 20-40%.
In Crohn's, a randomized controlled trial found that natalizumab increased the rate of remission [1].
Mechanism of action
The mechanism of action of natalizumab is believed to involve the inhibition of immune cells from crossing blood vessel walls to reach various tissues, including the brain.
Link to a rare brain disease
While the drug was shown to be powerfully effective for preventing relapses of MS, just three months after natalizumab was initially approved for MS by the FDA, the companies announced on February 28, 2005 that one fatal and one non-fatal case of a rare, often lethal brain disease known as progressive multifocal leukoencephalopathy (PML) were found in patients given natalizumab in combination with interferon beta-1a (Avonex) over a two year period.[2][3] Natalizumab was voluntarily withdrawn from the market that day,[4] after the first two confirmed cases, so that an intensive safety evaluation could be conducted to determine the prevalence of PML and what actions might be taken to minimize the likelihood of PML. During the safety review, a second PML death was attributed to natalizumab in March of 2005, in a Crohn’s disease patient who had died in December 2003 from what was thought at that time to be a brain tumor, but the diagnosis was subsequently re-evaluated as having been PML. The Crohn’s disease patient who had received eight doses of natalizumab over an 18-month period had a prior medication history which included multiple courses of immunosuppressant agents, which are thought to have contributed significantly to the PML.[5]
Biogen Idec and Élan were both affected financially by the reports of PML.
The companies announced in the fall of 2005 that they had resubmitted it to the U.S. Food and Drug Administration (FDA) and were also resubmitting to the European Medicines Agency (EMEA), for approval in Europe. The FDA again granted natalizumab "Priority Review" status, a designation granted to products considered to be potentially significant advancements over existing therapies. Under this designation, the FDA commits to render a decision within six months of submission rather than the standard ten months.
Return to the market
On June 5, 2006, after reviewing two years of safety and efficacy data and an Advisory Committee hearing ending in a unanimous recommendation for reapproval, FDA re-approved it for patients with relapsing forms of MS under certain conditions (the FDA Advisory Committee recommended the return of the treatment as a first line therapy but the FDA did not follow completely the recommendation).
They also included a special restricted distribution program known as the TOUCH Prescribing Program ("TOUCH" stands for "Tysabri Outreach: Unified Commitment to Health"). Under this program, only prescribers, infusion centers, and specialty pharmacies trained and enrolled in the TOUCH program can prescribe and administer Tysabri. Additionally, patients must also be enrolled in the TOUCH program, so that they may be educated on Tysabri as well as periodically evaluated while being treated with Tysabri.
In April of 2006 the Committee for Medicinal Products for Human Use (CHMP), the scientific committee of the European Medicines Agency (EMEA), issued a positive opinion recommending marketing authorization for natalizumab as a treatment for relapsing-remitting multiple sclerosis. Several weeks later on June 29, the EMEA also approved natalizumab in the European Union for relapsing forms of MS, but not subject to the TOUCH restrictions.[6]
Price and marketing issues
The wholesale price of Tysabri is US$2184.62 per vial. Given that natalizumab is generally administered 13 times a year, the annual costs for Tysabri is approximately US$28,400 (not including costs associated with infusion services), priced at a premium to the older generation drugs.
References
- ↑ Ghosh S, Goldin E, Gordon F, Malchow H, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S (2003). "Natalizumab for active Crohn's disease". N. Engl. J. Med. 348 (1): 24–32. PMID 12510039.
- ↑ Kleinschmidt-DeMasters BK, Tyler KL; Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005 Jul 28;353(4):369-74.
- ↑ Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D; Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005 Jul 28;353(4):375-81.
- ↑ "PHARMACEUTICALS: RESTRICTIONS IN USE AND AVAILABILITY" (PDF). World Health Organization. Retrieved 2007-07-23.
- ↑ Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts; Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med. 2005 Jul 28;353(4):362-8.
- ↑ Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue EW, Jager HR, Clifford DB. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006 Mar 2;354(9):924-33.
External links
- Tysabri Natalizumab
- Elan's Tysabri information center
- Biogen's Tysabri information center
- Site created by MS patients
Template:Immunosuppressants Template:Humanizedmonoclonals
de:Natalizumab he:טיסברי it:Natalizumab Template:WikiDoc Sources