Naratriptan adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Adverse Reactions
Serious cardiac events, including some that have been fatal, have occurred following the use of 5-HT1 agonists. These events are extremely rare and most have been reported in patients with risk factors predictive of CAD. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation (see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).
Incidence in Controlled Clinical Trials
The most common adverse events were paresthesias, dizziness, drowsiness, malaise/fatigue, and throat/neck symptoms, which occurred at a rate of 2% and at least 2 times placebo rate. Since patients treated only 1 to 3 headaches in the controlled clinical trials, the opportunity for discontinuation of therapy in response to an adverse event was limited. In a long-term, open-label study where patients were allowed to treat multiple migraine attacks for up to 1 year, 15 patients (3.6%) discontinued treatment due to adverse events.
Table 2 lists adverse events that occurred in 5 placebo-controlled clinical trials of approximately 1,752 exposures to placebo and naratriptan tablets in adult migraine patients.
The events cited reflect experience gained under closely monitored conditions of clinical trials in a highly selected patient population. In actual clinical practice or in other clinical trials, these frequency estimates may not apply, as the conditions of use, reporting behavior, and the kinds of patients treated may differ. Only events that occurred at a frequency of 2% or more in the group treated with naratriptan tablets 2.5 mg and were more frequent in that group than in the placebo group are included in Table 2. From this table, it appears that many of these adverse events are dose related.
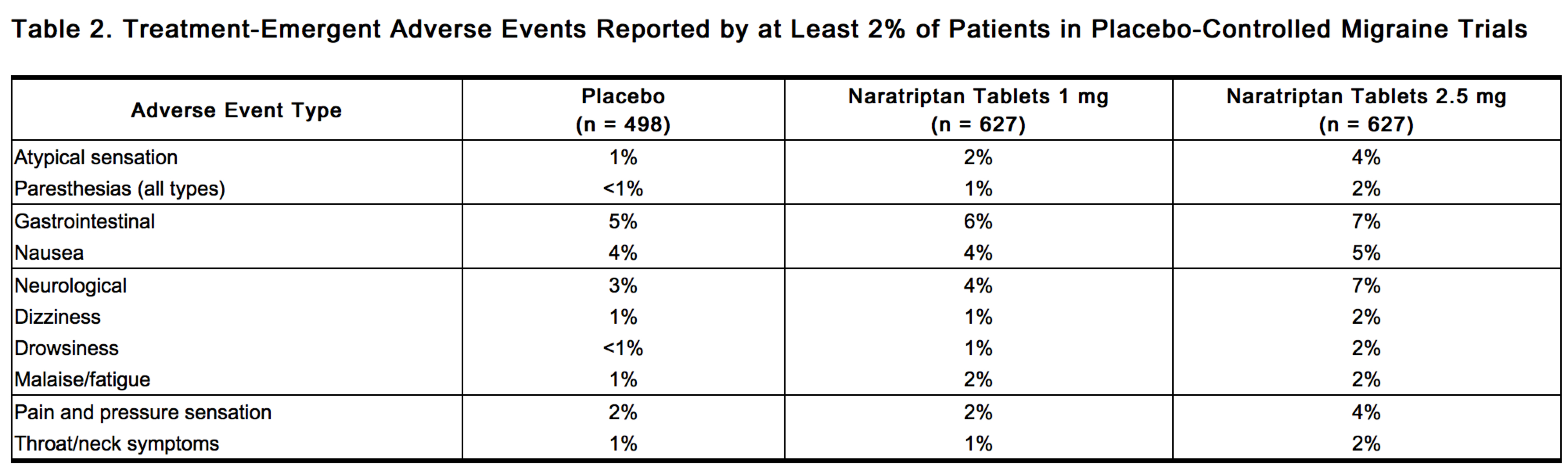 |
One event (vomiting) present in more than 1% of patients receiving naratriptan tablets occurred more frequently on placebo than on naratriptan 2.5 mg.
Naratriptan tablets are generally well tolerated. Most adverse reactions were mild and transient.
The incidence of adverse events in placebo-controlled clinical trials was not affected by age or weight of the patients, duration of headache prior to treatment, presence of aura, use of prophylactic medications, or tobacco use. There was insufficient data to assess the impact of race on the incidence of adverse events.
Other Events Observed in Association With the Administration of Naratriptan Tablets
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical events are presented. Because the reports include events observed in open and uncontrolled studies, the role of naratriptan tablets in their causation cannot be reliably determined. Furthermore, variability associated with adverse event reporting, the terminology used to describe adverse events, etc., limit the value of the quantitative frequency estimates provided. Event frequencies are calculated as the number of patients reporting an event divided by the total number of patients (n = 3,557) exposed to oral naratriptan doses up to 10 mg. All reported events are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are those occurring in at least 1/100 patients, infrequent adverse events are those occurring in 1/100 to 1/1,000 patients, and rare adverse events are those occurring in fewer than 1/1,000 patients.
Atypical Sensations
Frequent were warm/cold temperature sensations. Infrequent were feeling strange and burning/stinging sensation.
Cardiovascular
Infrequent were palpitations, increased blood pressure, tachyarrhythmias, and abnormal ECG (PR prolongation, QTc prolongation, ST/T wave abnormalities, premature ventricular contractions, atrial flutter, or atrial fibrillation), and syncope. Rare were bradycardia, varicosities, hypotension, and heart murmurs.
Ear, Nose, and Throat
Frequent were ear, nose, and throat infections. Infrequent were phonophobia, sinusitis, upper respiratory inflammation, and tinnitus. Rare were allergic rhinitis; labyrinthitis; ear, nose, and throat hemorrhage; and hearing difficulty.
Endocrine and Metabolic
Infrequent were thirst and polydipsia, dehydration, and fluid retention. Rare were hyperlipidemia, hypercholesterolemia, hypothyroidism, hyperglycemia, glycosuria and ketonuria, and parathyroid neoplasm.
Eye
Frequent was photophobia. Infrequent was blurred vision. Rare were eye pain and discomfort, sensation of eye pressure, eye hemorrhage, dry eyes, difficulty focusing, and scotoma.
Gastrointestinal
Frequent were hyposalivation and vomiting. Infrequent were dyspeptic symptoms, diarrhea, gastrointestinal discomfort and pain, gastroenteritis, and constipation. Rare were abnormal liver function tests, abnormal bilirubin levels, hemorrhoids, gastritis, esophagitis, salivary gland inflammation, oral itching and irritation, regurgitation and reflux, and gastric ulcers.
Hematological Disorders
Infrequent was increased white cells. Rare were thrombocytopenia, quantitative red cell or hemoglobin defects, anemia, and purpura.
Lower Respiratory Tract
Infrequent were bronchitis, cough, and pneumonia. Rare were tracheitis, asthma, pleuritis, and airway constriction and obstruction.
Musculoskeletal
Infrequent were muscle pain, arthralgia and articular rheumatism, muscle cramps and spasms, joint and muscle stiffness, tightness, and rigidity. Rare were bone and skeletal pain.
Neurological
Frequent was vertigo. Infrequent were tremors, cognitive function disorders, sleep disorders, and disorders of equilibrium. Rare were compressed nerve syndromes, confusion, sedation, hyperesthesia, coordination disorders, paralysis of cranial nerves, decreased consciousness, dreams, altered sense of taste, neuralgia, neuritis, aphasia, hypoesthesia, motor retardation, muscle twitching and fasciculation, psychomotor restlessness, and convulsions.
Non-Site Specific
Infrequent were chills and/or fever, descriptions of odor or taste, edema and swelling, allergies, and allergic reactions. Rare were spasms and mobility disorders.
Pain and Pressure Sensations
Frequent were pressure/tightness/heaviness sensations.
Psychiatry
Infrequent were anxiety, depressive disorders, and detachment. Rare were aggression and hostility, agitation, hallucinations, panic, and hyperactivity.
Reproduction
Rare were lumps of female reproductive tract, breast inflammation, inflammation of vagina, inflammation of fallopian tube, breast discharge, endometrium disorders, decreased libido, and lumps of breast.
Skin
Infrequent were sweating, skin rashes, pruritus, and urticaria. Rare were skin erythema, dermatitis and dermatosis, hair loss and alopecia, pruritic skin rashes, acne and folliculitis, allergic skin reactions, macular skin/rashes, skin photosensitivity, photodermatitis, skin flakiness, and dry skin.
Urology
Infrequent were bladder inflammation and polyuria and diuresis. Rare were urinary tract hemorrhage, urinary urgency, pyelitis, and urinary incontinence.
Observed During Clinical Practice
The following section enumerates potentially important adverse events that have occurred in clinical practice and that have been reported spontaneously to various surveillance systems. The events enumerated represent reports arising from both domestic and nondomestic use of naratriptan. These events do not include those already listed in the ADVERSE REACTIONS section above. Because the reports cite events reported spontaneously from worldwide postmarketing experience, frequency of events and the role of naratriptan in their causation cannot be reliably determined.
Cardiovascular
Angina, myocardial infarction (see WARNINGS).
Gastrointestinal
Colonic ischemia (see WARNINGS).
Lower Respiratory
Dyspnea.
Miscellaneous
Hypersensitivity, including anaphylaxis/anaphylactoid reactions, in some cases severe (e.g., circulatory collapse) (see WARNINGS).
Neurologic
Cerebral vascular accident, including transient ischemic attack, subarachnoid hemorrhage, and cerebral infarction (see WARNINGS); serotonin syndrome.
DRUG ABUSE AND DEPENDENCE
In one clinical study enrolling 12 subjects, all of whom had experience using oral opiates and other psychoactive drugs, naratriptan tablets produced less intense subjective responses ordinarily associated with many drugs of abuse than did codeine (30 to 90 mg).[1]
References
Adapted from the FDA Package Insert.