Naloxone: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} +, -{{EH}} +, -{{EJ}} +, -{{Editor Help}} +, -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag= | ||
| | |||
| | {{VP}} | ||
| | |||
| | <!--Overview--> | ||
|genericName= | |||
| | |||
| | |aOrAn= | ||
| | |||
| | an | ||
| | |drugClass= | ||
| | |||
| | [[opioid]] antagonist | ||
| | |||
}} | |indication= | ||
known or suspected [[opioid overdose]], as manifested by [[respiratory]] and/or [[central nervous system]] depression | |||
|hasBlackBoxWarning= | |||
|adverseReactions= | |||
[[hypotension]], [[hypertension]], [[ventricular tachycardia]], [[ventricular fibrillation]], [[dyspnea]], [[pulmonary edema]], [[cardiac arrest]], death, [[coma]], and [[encephalopathy]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition3===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition4===== | |||
* Dosing Information | |||
:* Dosage | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
*EVZIO is contraindicated in patients known to be [[hypersensitive]] to naloxone hydrochloride or to any of the other ingredients. | |||
<!--Warnings--> | |||
|warnings= | |||
====Precautions==== | |||
* Duration of Effect | |||
:*The duration of action of most opioids is likely to exceed that of EVZIO resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek immediate emergency medical assistance after delivering the first dose of EVZIO, keep the patient under continued surveillance, and repeat doses of EVZIO as necessary. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance. | |||
*Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists | |||
:*Reversal of respiratory depression by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete. Large doses of naloxone hydrochloride are required to antagonize buprenorphine because the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression. | |||
*Precipitation of Severe Opioid Withdrawal | |||
:*The use of EVZIO in patients who are opioid dependent may precipitate an acute abstinence syndrome characterized by the following signs and symptoms: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may include the following signs and symptoms: convulsions, excessive crying, and hyperactive reflexes. | |||
:*Abrupt postoperative reversal of opioid depression after using naloxone hydrochloride may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. These events have occurred in patients most of whom had pre-existing cardiovascular disorders or received other drugs which may have similar adverse cardiovascular effects. Although a direct cause and effect relationship has not been established, after use of naloxone hydrochloride, patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects should be monitored for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone hydrochloride is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*The following adverse reactions have been identified during post-approval use of naloxone hydrochloride in the post-operative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in post-operative patients have resulted in significant reversal of analgesia and have caused agitation [see Warnings and Precautions (5.3)]. | |||
*Abrupt reversal of opioid effects in persons who were physically dependent on opioids has precipitated an acute withdrawal syndrome. Signs and symptoms have included: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, tachycardia. In the neonate, opioid withdrawal signs and symptoms also included: convulsions, excessive crying, hyperactive reflexes [see Warnings and Precautions (5.3)]. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
* Drug | |||
:* Description | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
* Intravenous | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
* Description | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
* Description | |||
====Management==== | |||
* Description | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* | |||
<!--Structure--> | |||
|structure= | |||
* | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
= | |alcohol= | ||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
== | {{PillImage | ||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
= | {{LabelImage | ||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{ | {{LabelImage | ||
{{ | |fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | ||
}} | |||
<!--Category--> | |||
[[ | [[Category:Drug]] | ||
Revision as of 17:01, 15 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Naloxone is an opioid antagonist that is FDA approved for the {{{indicationType}}} of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. Common adverse reactions include hypotension, hypertension, ventricular tachycardia, ventricular fibrillation, dyspnea, pulmonary edema, cardiac arrest, death, coma, and encephalopathy.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Naloxone in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Naloxone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Naloxone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Naloxone in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Naloxone in pediatric patients.
Contraindications
- EVZIO is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or to any of the other ingredients.
Warnings
Precautions
- Duration of Effect
- The duration of action of most opioids is likely to exceed that of EVZIO resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek immediate emergency medical assistance after delivering the first dose of EVZIO, keep the patient under continued surveillance, and repeat doses of EVZIO as necessary. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
- Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
- Reversal of respiratory depression by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete. Large doses of naloxone hydrochloride are required to antagonize buprenorphine because the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression.
- Precipitation of Severe Opioid Withdrawal
- The use of EVZIO in patients who are opioid dependent may precipitate an acute abstinence syndrome characterized by the following signs and symptoms: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may include the following signs and symptoms: convulsions, excessive crying, and hyperactive reflexes.
- Abrupt postoperative reversal of opioid depression after using naloxone hydrochloride may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. These events have occurred in patients most of whom had pre-existing cardiovascular disorders or received other drugs which may have similar adverse cardiovascular effects. Although a direct cause and effect relationship has not been established, after use of naloxone hydrochloride, patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects should be monitored for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone hydrochloride is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions have been identified during post-approval use of naloxone hydrochloride in the post-operative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in post-operative patients have resulted in significant reversal of analgesia and have caused agitation [see Warnings and Precautions (5.3)].
- Abrupt reversal of opioid effects in persons who were physically dependent on opioids has precipitated an acute withdrawal syndrome. Signs and symptoms have included: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, tachycardia. In the neonate, opioid withdrawal signs and symptoms also included: convulsions, excessive crying, hyperactive reflexes [see Warnings and Precautions (5.3)].
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Naloxone in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Naloxone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Naloxone during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Naloxone with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Naloxone with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Naloxone with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Naloxone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Naloxone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Naloxone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Naloxone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Naloxone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Naloxone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Naloxone in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Naloxone in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Naloxone in the drug label.
Pharmacology
There is limited information regarding Naloxone Pharmacology in the drug label.
Mechanism of Action
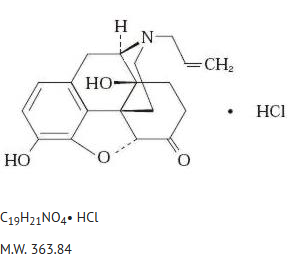
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Naloxone in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Naloxone in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Naloxone in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Naloxone in the drug label.
How Supplied
Storage
There is limited information regarding Naloxone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Naloxone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Naloxone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Naloxone in the drug label.
Precautions with Alcohol
- Alcohol-Naloxone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Naloxone |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Naloxone |Label Name=Naloxone11.png
}}
{{#subobject:
|Label Page=Naloxone |Label Name=Naloxone11.png
}}