Naldemedine: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 3: | Line 3: | ||
|genericName=generic name | |genericName=generic name | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass= | |drugClass=[[opioid]] [[antagonist]] | ||
|indicationType= | |indicationType=treatment | ||
|indication= | |indication=opioid-induced [[constipation]] (OIC) in adult patients with [[chronic]] non-cancer pain, including patients with chronic pain related to prior [[cancer]] or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation | ||
|adverseReactions= | |adverseReactions=[[abdominal pain]], [[diarrhea]], [[nausea]], and [[gastroenteritis]] | ||
|fdaLIADAdult======Indications:===== | |fdaLIADAdult======Indications:===== | ||
* | *Naldemedine is indicated for the treatment of opioid-induced constipation (OIC) in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation. | ||
=====Adult Dosage:===== | =====Adult Dosage:===== | ||
*The recommended dosage of | *The recommended dosage of Naldemedine is 0.2 mg orally once daily with or without food. | ||
|offLabelAdultGuideSupport=There is limited information regarding Naldemedine ''Off-Label Guideline-Supported Use and Dosage (Adult)'' in the drug label. | |offLabelAdultGuideSupport=There is limited information regarding Naldemedine ''Off-Label Guideline-Supported Use and Dosage (Adult)'' in the drug label. | ||
| Line 27: | Line 27: | ||
|contraindications=* | |contraindications=*Naldemedine is contraindicated in: | ||
:*Patients with known or suspected gastrointestinal obstruction and patients at increased risk of recurrent obstruction, due to the potential for gastrointestinal perforation. | :*Patients with known or suspected gastrointestinal obstruction and patients at increased risk of recurrent obstruction, due to the potential for gastrointestinal perforation. | ||
:*Patients with a history of a hypersensitivity reaction to | :*Patients with a history of a hypersensitivity reaction to Naldemedine. Reactions have included bronchospasm and rash. | ||
|warnings======Gastrointestinal Perforation===== | |warnings======Gastrointestinal Perforation===== | ||
*Cases of gastrointestinal perforation have been reported with use of another peripherally acting opioid antagonist in patients with conditions that may be associated with localized or diffuse reduction of structural integrity in the wall of the gastrointestinal tract (e.g., peptic ulcer disease, Ogilvie’s syndrome, diverticular disease, infiltrative gastrointestinal tract malignancies, or peritoneal metastases). Take into account the overall risk-benefit profile when using | *Cases of gastrointestinal perforation have been reported with use of another peripherally acting opioid antagonist in patients with conditions that may be associated with localized or diffuse reduction of structural integrity in the wall of the gastrointestinal tract (e.g., peptic ulcer disease, Ogilvie’s syndrome, diverticular disease, infiltrative gastrointestinal tract malignancies, or peritoneal metastases). Take into account the overall risk-benefit profile when using Naldemedine in patients with these conditions or other conditions which might result in impaired integrity of the gastrointestinal tract wall (e.g., Crohn’s disease). Monitor for the development of severe, persistent, or worsening abdominal pain; discontinue Naldemedine in patients who develop this symptom. | ||
=====Opioid Withdrawal===== | =====Opioid Withdrawal===== | ||
*Clusters of symptoms consistent with opioid withdrawal, including hyperhidrosis, chills, increased lacrimation, hot flush/flushing, pyrexia, sneezing, feeling cold, abdominal pain, diarrhea, nausea, and vomiting have occurred in patients treated with | *Clusters of symptoms consistent with opioid withdrawal, including hyperhidrosis, chills, increased lacrimation, hot flush/flushing, pyrexia, sneezing, feeling cold, abdominal pain, diarrhea, nausea, and vomiting have occurred in patients treated with Naldemedine. | ||
*Patients having disruptions to the blood-brain barrier may be at increased risk for opioid withdrawal or reduced analgesia. Take into account the overall risk-benefit profile when using | *Patients having disruptions to the blood-brain barrier may be at increased risk for opioid withdrawal or reduced analgesia. Take into account the overall risk-benefit profile when using Naldemedine in such patients. Monitor for symptoms of opioid withdrawal in such patients. | ||
|clinicalTrials=*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
*The data described below reflect exposure to | *The data described below reflect exposure to Naldemedine in 1163 patients in clinical trials, including 487 patients with exposures greater than six months and 203 patients with exposures of 12 months. | ||
*The following safety data are derived from three double-blind, placebo-controlled trials in patients with OIC and chronic non-cancer pain: two 12-week studies (Studies 1 and 2) and one 52-week study (Study 3). | *The following safety data are derived from three double-blind, placebo-controlled trials in patients with OIC and chronic non-cancer pain: two 12-week studies (Studies 1 and 2) and one 52-week study (Study 3). | ||
*In Studies 1 and 2, patients on laxatives were required to discontinue their use prior to study enrollment. All patients were restricted to bisacodyl rescue treatment during the study. In Study 3, approximately 60% of patients in both treatment groups were on a laxative regimen at baseline; patients were allowed to continue using their laxative regimen throughout the study duration. The safety profile of | *In Studies 1 and 2, patients on laxatives were required to discontinue their use prior to study enrollment. All patients were restricted to bisacodyl rescue treatment during the study. In Study 3, approximately 60% of patients in both treatment groups were on a laxative regimen at baseline; patients were allowed to continue using their laxative regimen throughout the study duration. The safety profile of Naldemedine relative to placebo was similar regardless of laxative use. | ||
*Tables 1 and 2 list common adverse reactions occurring in at least 2% of patients receiving | *Tables 1 and 2 list common adverse reactions occurring in at least 2% of patients receiving Naldemedine and at an incidence greater than placebo. Table 1 shows pooled 12-week data from Studies 1 and 2. Table 2 shows 12-week data from Study 3. | ||
[[image:Naldemedine_Adverse_Reactions_Tables_1_and_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Naldemedine_Adverse_Reactions_Tables_1_and_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
*Adverse reactions up to 12 months in Study 3 are similar to those listed in Tables 1 and 2 (diarrhea: 11% vs. 5%, abdominal pain: 8% vs. 3%, and nausea: 8% vs. 6% for | *Adverse reactions up to 12 months in Study 3 are similar to those listed in Tables 1 and 2 (diarrhea: 11% vs. 5%, abdominal pain: 8% vs. 3%, and nausea: 8% vs. 6% for Naldemedine and placebo, respectively). | ||
Opioid Withdrawal | Opioid Withdrawal | ||
| Line 63: | Line 63: | ||
*Adverse reactions of possible opioid withdrawal could include non-gastrointestinal (GI) symptoms (e.g., hyperhidrosis, hot flush or flushing, chills, tremor, tachycardia, anxiety, agitation, yawning, rhinorrhea, increased lacrimation, sneezing, feeling cold, and pyrexia), GI symptoms (e.g., vomiting, diarrhea, or abdominal pain), or both GI and non-GI symptoms. | *Adverse reactions of possible opioid withdrawal could include non-gastrointestinal (GI) symptoms (e.g., hyperhidrosis, hot flush or flushing, chills, tremor, tachycardia, anxiety, agitation, yawning, rhinorrhea, increased lacrimation, sneezing, feeling cold, and pyrexia), GI symptoms (e.g., vomiting, diarrhea, or abdominal pain), or both GI and non-GI symptoms. | ||
*In pooled Studies 1 and 2, the incidence of adverse reactions of opioid withdrawal was 1% (8/542) for | *In pooled Studies 1 and 2, the incidence of adverse reactions of opioid withdrawal was 1% (8/542) for Naldemedine and 1% (3/546) for placebo. In Study 3 (52-week data), the incidence was 3% (20/621) for Naldemedine and 1% (9/619) for placebo. Most Naldemedine treated subjects experienced nearly equal incidence of GI only or both GI and non-GI symptoms. | ||
Less Common Adverse Reactions: | Less Common Adverse Reactions: | ||
*Two patients developed symptoms of hypersensitivity following a single dose of | *Two patients developed symptoms of hypersensitivity following a single dose of Naldemedine. One patient reported bronchospasm and another rash. | ||
|postmarketing= | |postmarketing= | ||
|drugInteractions=*Table 3 includes drugs with clinically important drug interactions with | |drugInteractions=*Table 3 includes drugs with clinically important drug interactions with Naldemedine and instructions for preventing or managing the interaction. | ||
[[image:Naldemedine_Drug_Interactions_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Naldemedine_Drug_Interactions_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 77: | Line 77: | ||
|useInPregnancyFDA=Risk Summary | |useInPregnancyFDA=Risk Summary | ||
*There are no available data with | *There are no available data with Naldemedine in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. There is a potential for opioid withdrawal in a fetus when Naldemedine is used in pregnant women. Naldemedine should be used during pregnancy only if the potential benefit justifies the potential risk. | ||
*In a rat embryo-fetal development study following oral administration of | *In a rat embryo-fetal development study following oral administration of Naldemedine during the period of organogenesis at doses resulting in systemic exposure approximately 23,000 times the human area under the plasma-concentration time curve (AUC) at the recommended human dose of 0.2 mg/day, no developmental abnormalities were observed. In rabbits, there were no adverse effects on embryo-fetal development following oral administration of Naldemedine during the period of organogenesis at doses resulting in systemic exposure approximately 226 times the human AUC at the recommended human dose of 0.2 mg/day. No effects on pre- and postnatal development were observed in rats at exposures 12 times human exposures at the recommended human dose. | ||
*The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. | *The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. | ||
| Line 91: | Line 91: | ||
Data (Animal) | Data (Animal) | ||
*In rats, there were no adverse effects on embryo-fetal development following oral administration of | *In rats, there were no adverse effects on embryo-fetal development following oral administration of Naldemedine during the period of organogenesis at doses up to 1000 mg/kg/day (approximately 23,000 times the human exposures (AUC) at the recommended human dose). In rabbits, there were no adverse effects on embryo-fetal development following oral administration of Naldemedine during the period of organogenesis at doses up to 100 mg/kg/day (approximately 226 times the human exposures (AUC) at the recommended human dose). At 400 mg/kg/day (approximately 844 times the human exposures (AUC) at the recommended human dose), effects in maternal animals included body weight loss/decreased body weight gain and food consumption, fetal loss, and premature delivery. Decreased fetal body weights at this dose may be related to the maternal toxicity observed. | ||
*In the pre- and postnatal development study, pregnant rats were administered | *In the pre- and postnatal development study, pregnant rats were administered Naldemedine at oral doses up to 1000 mg/kg/day from gestation day 7 through lactation day 20. No effects on pre- and postnatal development were observed in rats at 1 mg/kg/day (approximately 12 times the human exposures (AUC) at the recommended human dose). A single dam died at parturition at 1000 mg/kg/day, and decreased body weights/body weight gain and food consumption, poor nursing, and total litter loss were noted at 30 and 1000 mg/kg/day (approximately 626 and 17,000 times the human exposures (AUC) at the recommended human dose, respectively). Decreases in the offspring viability index on Day 4 after birth were noted at 30 and 1000 mg/kg/day, and low body weights and delayed pinna unfolding in pups were noted at 1000 mg/kg/day. | ||
|useInLaborDelivery= | |useInLaborDelivery= | ||
|useInNursing=Risk Summary | |useInNursing=Risk Summary | ||
*There is no information regarding the presence of | *There is no information regarding the presence of Naldemedine in human milk, the effects on the breastfed infant, or the effects on milk production. Naldemedine was present in the milk of rats. Because of the potential for serious adverse reactions, including opioid withdrawal in breastfed infants, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother. If drug is discontinued in order to minimize drug exposure to a breastfed infant, advise women that breastfeeding may be resumed 3 days after the final dose of Naldemedine. | ||
Data | Data | ||
*Drug-related radioactivity was transferred into milk of lactating rats following a single oral dose of 1 mg/kg [carbonyl-14C]- | *Drug-related radioactivity was transferred into milk of lactating rats following a single oral dose of 1 mg/kg [carbonyl-14C]-Naldemedine. | ||
|useInPed=*The safety and effectiveness of | |useInPed=*The safety and effectiveness of Naldemedine have not been established in pediatric patients. | ||
|useInGeri=*Of 1163 patients in clinical studies exposed to | |useInGeri=*Of 1163 patients in clinical studies exposed to Naldemedine, 183 (16%) were 65 years of age and over, while 37 (3%) were 75 years and over. No overall differences in safety or effectiveness between these and younger patients were observed, but greater sensitivity of some older individuals cannot be ruled out. In a population pharmacokinetic analysis, no age-related alterations in the pharmacokinetics of Naldemedine were observed. | ||
|useInGender= | |useInGender= | ||
|useInRace= | |useInRace= | ||
|useInRenalImpair= | |useInRenalImpair= | ||
|useInHepaticImpair=*The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of | |useInHepaticImpair=*The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of Naldemedine has not been evaluated. Avoid use of Naldemedine in patients with severe hepatic impairment. No dose adjustment of Naldemedine is required in patients with mild or moderate hepatic impairment. | ||
|useInReproPotential= | |useInReproPotential= | ||
|useInImmunocomp= | |useInImmunocomp= | ||
|administration=*Alteration of analgesic dosing regimen prior to initiating | |administration=*Alteration of analgesic dosing regimen prior to initiating Naldemedine is not required. | ||
*Patients receiving opioids for less than 4 weeks may be less responsive to | *Patients receiving opioids for less than 4 weeks may be less responsive to Naldemedine. | ||
*Discontinue | *Discontinue Naldemedine if treatment with the opioid pain medication is also discontinued. | ||
|monitoring=*Increased frequency of spontaneous bowel movements is indicative of efficacy. | |monitoring=*Increased frequency of spontaneous bowel movements is indicative of efficacy. | ||
| Line 128: | Line 128: | ||
*Symptoms of opioid withdrawal. | *Symptoms of opioid withdrawal. | ||
|overdose=*Single doses of | |overdose=*Single doses of Naldemedine up to 100 mg (500 times the recommended dose) and multiple doses of up to 30 mg (150 times the recommended dose) for 10 days have been administered to healthy subjects in clinical studies. Dose-dependent increases in gastrointestinal-related adverse reactions, including abdominal pain, diarrhea, and nausea, were observed. | ||
*Single doses of | *Single doses of Naldemedine up to 3 mg (15 times the recommended dose) and multiple doses of 0.4 mg (twice the recommended dose) for 28 days have been administered to patients with OIC in clinical studies. Dose-dependent increases in gastrointestinal-related adverse reactions, including abdominal pain, diarrhea, nausea, and vomiting, were observed. Also, chills, hyperhidrosis, and dizziness were reported more frequently at 1 and 3 mg doses and hyperhidrosis at the 0.4 mg dose. | ||
*No antidote for | *No antidote for Naldemedine is known. Hemodialysis is not an effective means to remove Naldemedine from the blood. | ||
|drugBox= | |drugBox= | ||
| Line 166: | Line 166: | ||
*Naldemedine is a derivative of naltrexone to which a side chain has been added that increases the molecular weight and the polar surface area, thereby reducing its ability to cross the blood-brain barrier (BBB). | *Naldemedine is a derivative of naltrexone to which a side chain has been added that increases the molecular weight and the polar surface area, thereby reducing its ability to cross the blood-brain barrier (BBB). | ||
*Naldemedine is also a substrate of the P-glycoprotein (P-gp) efflux transporter. Based on these properties, the CNS penetration of | *Naldemedine is also a substrate of the P-glycoprotein (P-gp) efflux transporter. Based on these properties, the CNS penetration of Naldemedine is expected to be negligible at the recommended dose levels, limiting the potential for interference with centrally-mediated opioid analgesia. | ||
|structure=[[image:Naldemedine_Structural_Formula.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |structure=[[image:Naldemedine_Structural_Formula.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
|PD=*Use of opioids induces slowing of gastrointestinal motility and transit. Antagonism of gastrointestinal mu-opioid receptors by | |PD=*Use of opioids induces slowing of gastrointestinal motility and transit. Antagonism of gastrointestinal mu-opioid receptors by Naldemedine inhibits opioid-induced delay of gastrointestinal transit time. | ||
Effect on Cardiac Repolarization | Effect on Cardiac Repolarization | ||
*At a dose up to 5-times the recommended dose, | *At a dose up to 5-times the recommended dose, Naldemedine does not prolong the QT interval to any clinically relevant extent. | ||
|PK=Absorption | |PK=Absorption | ||
*Following oral administration, | *Following oral administration, Naldemedine is absorbed with the time to achieve peak concentrations (Tmax) of approximately 0.75 hours in a fasted state. Across the range of doses evaluated, the maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) increased in a dose-proportional or almost dose-proportional manner. Accumulation was minimal following multiple daily doses of Naldemedine. | ||
''Food Effect'' | ''Food Effect'' | ||
*A high-fat meal decreased the rate, but not the extent of | *A high-fat meal decreased the rate, but not the extent of Naldemedine absorption. The Cmax was decreased by approximately 35% and time to achieve Cmax was delayed from 0.75 hours in the fasted state to 2.5 hours in the fed state, whereas there was no meaningful change in the AUC in the fed state. | ||
Distribution | Distribution | ||
*Plasma protein binding of | *Plasma protein binding of Naldemedine in humans is 93% to 94%. The mean apparent volume of distribution during the terminal phase (Vz/F) is 155 L. | ||
Elimination | Elimination | ||
*The terminal elimination half-life of | *The terminal elimination half-life of Naldemedine is 11 hours. | ||
''Metabolism'' | ''Metabolism'' | ||
*Naldemedine is primarily metabolized by CYP3A to nor- | *Naldemedine is primarily metabolized by CYP3A to nor-Naldemedine, with minor contribution from UGT1A3 to form Naldemedine 3-G. Nor-Naldemedine and Naldemedine 3-G have been shown to have antagonistic activity for opioid receptors, with less potent effect than Naldemedine. | ||
*Following oral administration of [14C]-labeled | *Following oral administration of [14C]-labeled Naldemedine, the primary metabolite in plasma was nor-Naldemedine, with a relative exposure compared to Naldemedine of approximately 9% to 13%. Naldemedine 3-G was a minor metabolite in plasma, with a relative exposure to Naldemedine of less than 3%. | ||
*Naldemedine also undergoes cleavage in the GI tract to form benzamidine and | *Naldemedine also undergoes cleavage in the GI tract to form benzamidine and Naldemedine carboxylic acid. | ||
''Excretion'' | ''Excretion'' | ||
*Following oral administration of [14C]-labeled | *Following oral administration of [14C]-labeled Naldemedine, the total amount of radioactivity excreted in the urine and feces was 57% and 35% of the administered dose of Naldemedine, respectively. The amount of Naldemedine excreted unchanged in the urine was approximately 16% to 18% of the administered dose. Benzamidine was the most predominant metabolite excreted in the urine and feces, representing approximately 32% and 20% of the administered dose of Naldemedine, respectively. The percentage of unchanged drug in feces has not been estimated. | ||
Use in Specific Populations | Use in Specific Populations | ||
| Line 208: | Line 208: | ||
''Age: Geriatric Population, Sex, Race/Ethnicity'' | ''Age: Geriatric Population, Sex, Race/Ethnicity'' | ||
*A population pharmacokinetic analysis from clinical studies with | *A population pharmacokinetic analysis from clinical studies with Naldemedine did not identify a clinically meaningful effect of age, sex, or race on the pharmacokinetics of Naldemedine. | ||
''Renal Impairment'' | ''Renal Impairment'' | ||
*The pharmacokinetics of | *The pharmacokinetics of Naldemedine after administration of a 0.2 mg single oral dose of Naldemedine was studied in 8 subjects with mild (n=8, estimated glomerular filtration rate [eGFR] of 60 to 89 mL/min/1.73 m2), moderate (n=8, eGFR 30 to 59 mL/min/1.73 m2), and severe (n=6, eGFR less than 30 mL/min/1.73 m2) renal impairment, and subjects with end-stage renal disease (ESRD) requiring hemodialysis (n=8), and compared to healthy subjects with normal renal function (n=8, estimated creatinine clearance of at least 90 mL/min). The pharmacokinetics of Naldemedine between subjects in all groups were similar. | ||
*Plasma concentrations of | *Plasma concentrations of Naldemedine in subjects with ESRD requiring hemodialysis were similar when Naldemedine was administered either pre- or post-hemodialysis, indicating that Naldemedine was not removed from the blood by hemodialysis. | ||
''Hepatic Impairment'' | ''Hepatic Impairment'' | ||
*The effect of hepatic impairment on the pharmacokinetics of a 0.2 mg single oral dose of | *The effect of hepatic impairment on the pharmacokinetics of a 0.2 mg single oral dose of Naldemedine was studied in subjects with hepatic impairment classified as mild (n=8, Child-Pugh Class A) or moderate (n=8, Child-Pugh Class B) and compared with healthy subjects with normal hepatic function (n=8). The pharmacokinetics of Naldemedine between subjects in all groups were similar. | ||
*The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of | *The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of Naldemedine was not evaluated. | ||
Drug Interaction Studies | Drug Interaction Studies | ||
| Line 226: | Line 226: | ||
''Effect of Naldemedine on Other Drugs'' | ''Effect of Naldemedine on Other Drugs'' | ||
*In in vitro studies at clinically relevant concentrations, | *In in vitro studies at clinically relevant concentrations, Naldemedine did not inhibit the major CYP enzymes (including CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or CYP4A11 isozymes) and is not an inhibitor of transporters (including OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, BCRP, or P-gp). Naldemedine did not cause significant induction of CYP1A2, CYP2B6, CYP3A4, UGT1A2, UGT1A6, or UGT2B7 isozymes. | ||
''Effect of Other Drugs on Naldemedine'' | ''Effect of Other Drugs on Naldemedine'' | ||
*Naldemedine is primarily metabolized by CYP3A4 enzyme with minor contribution from UGT1A3. Naldemedine is a substrate of P-gp. The effects of co-administered drugs on the pharmacokinetics of | *Naldemedine is primarily metabolized by CYP3A4 enzyme with minor contribution from UGT1A3. Naldemedine is a substrate of P-gp. The effects of co-administered drugs on the pharmacokinetics of Naldemedine are summarized in Figure 1. | ||
[[image:Naldemedine_Pharmacokinetics_Figure.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Naldemedine_Pharmacokinetics_Figure.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 238: | Line 238: | ||
Carcinogenesis | Carcinogenesis | ||
*In 2-year carcinogenicity studies, there were no drug-related neoplastic findings following oral administration of | *In 2-year carcinogenicity studies, there were no drug-related neoplastic findings following oral administration of Naldemedine to mice and rats at doses up to 100 mg/kg/day (approximately 17,500 and 6,300 times the human exposures (AUC) at the recommended human dose, respectively). | ||
Mutagenesis | Mutagenesis | ||
| Line 248: | Line 248: | ||
*Naldemedine was found to have no effect on fertility or reproductive performance in male and female rats at oral doses up to 1000 mg/kg/day (approximately 17,000 times the human exposures (AUC) at the recommended human dose). In female rats, prolongation of diestrous phase was noted at 10 mg/kg/day (approximately 179 times the human exposures (AUC) at the recommended human dose). | *Naldemedine was found to have no effect on fertility or reproductive performance in male and female rats at oral doses up to 1000 mg/kg/day (approximately 17,000 times the human exposures (AUC) at the recommended human dose). In female rats, prolongation of diestrous phase was noted at 10 mg/kg/day (approximately 179 times the human exposures (AUC) at the recommended human dose). | ||
|clinicalStudies=* | |clinicalStudies=*Naldemedine was evaluated in two replicate, 12-week, randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) in which Naldemedine was used without laxatives in patients with OIC and chronic non-cancer pain. | ||
*Patients receiving a stable opioid morphine equivalent daily dose of at least 30 mg for at least 4 weeks before enrollment and self-reported OIC were eligible for clinical trial participation. | *Patients receiving a stable opioid morphine equivalent daily dose of at least 30 mg for at least 4 weeks before enrollment and self-reported OIC were eligible for clinical trial participation. | ||
| Line 262: | Line 262: | ||
*In the screening and treatment periods, bisacodyl was used as rescue laxative if patients had not had a BM for 72 hours and were allowed one-time use of an enema, if after 24 hours of taking bisacodyl they still had not had a BM. | *In the screening and treatment periods, bisacodyl was used as rescue laxative if patients had not had a BM for 72 hours and were allowed one-time use of an enema, if after 24 hours of taking bisacodyl they still had not had a BM. | ||
*A total of 547 patients in Study 1 and 553 patients in Study 2 were randomized in a 1:1 ratio to receive | *A total of 547 patients in Study 1 and 553 patients in Study 2 were randomized in a 1:1 ratio to receive Naldemedine 0.2 mg once daily or placebo for 12 weeks. Study medication was administered without regard to meals. | ||
*The mean age of subjects in Studies 1 and 2 was 54 years; 59% were women; and 80% were white. The most common types of pain in Studies 1 and 2 were back or neck pain (61%). The mean baseline number of SBMs was 1.3 and 1.2 per week for Studies 1 and 2, respectively. | *The mean age of subjects in Studies 1 and 2 was 54 years; 59% were women; and 80% were white. The most common types of pain in Studies 1 and 2 were back or neck pain (61%). The mean baseline number of SBMs was 1.3 and 1.2 per week for Studies 1 and 2, respectively. | ||
| Line 268: | Line 268: | ||
*Prior to enrollment, patients were using their current opioid for a mean duration of approximately 5 years. A wide range of types of opioids were used. The mean baseline opioid morphine equivalent daily dosage was 132 mg and 121 mg per day for Studies 1 and 2, respectively. | *Prior to enrollment, patients were using their current opioid for a mean duration of approximately 5 years. A wide range of types of opioids were used. The mean baseline opioid morphine equivalent daily dosage was 132 mg and 121 mg per day for Studies 1 and 2, respectively. | ||
*The efficacy of | *The efficacy of Naldemedine was assessed in Studies 1 and 2 using a responder analysis. A responder was defined as a patient who had at least 3 SBMs per week and a change from baseline of at least 1 SBM per week for at least 9 out of the 12 weeks and 3 out of the last 4 weeks in Studies 1 and 2. | ||
*The responder rates in Studies 1 and 2 are shown in Table 4. | *The responder rates in Studies 1 and 2 are shown in Table 4. | ||
| Line 274: | Line 274: | ||
[[image:Naldemedine_Clinical_Studies_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Naldemedine_Clinical_Studies_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
*In Studies 1 and 2, the mean increase in frequency of SBMs per week from baseline to the last 2 weeks of the 12-week treatment period was 3.1 for | *In Studies 1 and 2, the mean increase in frequency of SBMs per week from baseline to the last 2 weeks of the 12-week treatment period was 3.1 for Naldemedine vs. 2.0 for placebo (difference 1.0, 95% CI 0.6, 1.5), and 3.3 for Naldemedine vs. 2.1 for placebo (difference 1.2, 95% CI 0.8, 1.7), respectively. | ||
*During week 1 of the treatment period, the mean increase in frequency of SBMs per week from baseline was 3.3 for | *During week 1 of the treatment period, the mean increase in frequency of SBMs per week from baseline was 3.3 for Naldemedine vs. 1.3 for placebo (difference 2.0, 95% CI 1.5, 2.5) in Study 1 and 3.7 for Naldemedine vs. 1.6 for placebo (difference 2.1, 95% CI 1.5, 2.6) in Study 2. | ||
*The mean increase in the frequency of complete SBM (CSBM) per week from baseline to the last 2 weeks of 12-week treatment period was 2.3 for | *The mean increase in the frequency of complete SBM (CSBM) per week from baseline to the last 2 weeks of 12-week treatment period was 2.3 for Naldemedine vs. 1.5 for placebo (difference 0.8, 95% CI 0.4, 1.2) in Study 1 and 2.6 for Naldemedine vs. 1.6 for placebo (difference 1.1, 95% CI 0.6, 1.5) in Study 2. A CSBM was defined as a SBM that was associated with a sense of complete evacuation. | ||
*The change in the frequency of SBMs without straining per week from baseline to the last 2 weeks of the treatment period was 1.3 for | *The change in the frequency of SBMs without straining per week from baseline to the last 2 weeks of the treatment period was 1.3 for Naldemedine vs. 0.7 for placebo (difference 0.6, 95% CI 0.2, 0.9) in Study 1 and 1.8 for Naldemedine vs. 1.1 for placebo (difference 0.7, 95% CI 0.3, 1.2) in Study 2. | ||
|howSupplied=* | |howSupplied=*Naldemedine is supplied as 0.2 mg Naldemedine tablets in: | ||
:*bottle of 30 tablets - NDC 59011-523-30 | :*bottle of 30 tablets - NDC 59011-523-30 | ||
| Line 288: | Line 288: | ||
:*bottle of 90 tablets - NDC 59011-523-90 | :*bottle of 90 tablets - NDC 59011-523-90 | ||
|storage=*Store | |storage=*Store Naldemedine in light resistant container at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). | ||
|packLabel=[[image:Naldemedine_Package_Label_1.jpeg|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |packLabel=[[image:Naldemedine_Package_Label_1.jpeg|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 300: | Line 300: | ||
|fdaPatientInfo=Administration | |fdaPatientInfo=Administration | ||
*Advise patients to discontinue | *Advise patients to discontinue Naldemedine if treatment with the opioid pain medication is also discontinued. | ||
Gastrointestinal Perforation | Gastrointestinal Perforation | ||
*Advise patients to discontinue | *Advise patients to discontinue Naldemedine and to promptly seek medical attention if they develop unusually severe, persistent or worsening abdominal pain. | ||
Opioid Withdrawal | Opioid Withdrawal | ||
*Advise patients that clusters of symptoms consistent with opioid withdrawal may occur while taking | *Advise patients that clusters of symptoms consistent with opioid withdrawal may occur while taking Naldemedine and to contact their healthcare provider if these symptoms occur. | ||
Pregnancy | Pregnancy | ||
*Advise females of reproductive potential, who become pregnant or are planning to become pregnant, that the use of | *Advise females of reproductive potential, who become pregnant or are planning to become pregnant, that the use of Naldemedine during pregnancy may precipitate opioid withdrawal in a fetus due to the undeveloped blood-brain barrier. | ||
Lactation | Lactation | ||
*Advise women that breastfeeding is not recommended during treatment with | *Advise women that breastfeeding is not recommended during treatment with Naldemedine and for 3 days after the final dose. | ||
[[image:Naldemedine_Patient_Counseling_Information.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Naldemedine_Patient_Counseling_Information.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
Latest revision as of 13:42, 20 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Naldemedine is a opioid antagonist that is FDA approved for the treatment of opioid-induced constipation (OIC) in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation. Common adverse reactions include abdominal pain, diarrhea, nausea, and gastroenteritis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Naldemedine is indicated for the treatment of opioid-induced constipation (OIC) in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation.
Adult Dosage:
- The recommended dosage of Naldemedine is 0.2 mg orally once daily with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Naldemedine Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Naldemedine Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Naldemedine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Naldemedine Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Naldemedine Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Naldemedine is contraindicated in:
- Patients with known or suspected gastrointestinal obstruction and patients at increased risk of recurrent obstruction, due to the potential for gastrointestinal perforation.
- Patients with a history of a hypersensitivity reaction to Naldemedine. Reactions have included bronchospasm and rash.
Warnings
Gastrointestinal Perforation
- Cases of gastrointestinal perforation have been reported with use of another peripherally acting opioid antagonist in patients with conditions that may be associated with localized or diffuse reduction of structural integrity in the wall of the gastrointestinal tract (e.g., peptic ulcer disease, Ogilvie’s syndrome, diverticular disease, infiltrative gastrointestinal tract malignancies, or peritoneal metastases). Take into account the overall risk-benefit profile when using Naldemedine in patients with these conditions or other conditions which might result in impaired integrity of the gastrointestinal tract wall (e.g., Crohn’s disease). Monitor for the development of severe, persistent, or worsening abdominal pain; discontinue Naldemedine in patients who develop this symptom.
Opioid Withdrawal
- Clusters of symptoms consistent with opioid withdrawal, including hyperhidrosis, chills, increased lacrimation, hot flush/flushing, pyrexia, sneezing, feeling cold, abdominal pain, diarrhea, nausea, and vomiting have occurred in patients treated with Naldemedine.
- Patients having disruptions to the blood-brain barrier may be at increased risk for opioid withdrawal or reduced analgesia. Take into account the overall risk-benefit profile when using Naldemedine in such patients. Monitor for symptoms of opioid withdrawal in such patients.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure to Naldemedine in 1163 patients in clinical trials, including 487 patients with exposures greater than six months and 203 patients with exposures of 12 months.
- The following safety data are derived from three double-blind, placebo-controlled trials in patients with OIC and chronic non-cancer pain: two 12-week studies (Studies 1 and 2) and one 52-week study (Study 3).
- In Studies 1 and 2, patients on laxatives were required to discontinue their use prior to study enrollment. All patients were restricted to bisacodyl rescue treatment during the study. In Study 3, approximately 60% of patients in both treatment groups were on a laxative regimen at baseline; patients were allowed to continue using their laxative regimen throughout the study duration. The safety profile of Naldemedine relative to placebo was similar regardless of laxative use.
- Tables 1 and 2 list common adverse reactions occurring in at least 2% of patients receiving Naldemedine and at an incidence greater than placebo. Table 1 shows pooled 12-week data from Studies 1 and 2. Table 2 shows 12-week data from Study 3.

- Adverse reactions up to 12 months in Study 3 are similar to those listed in Tables 1 and 2 (diarrhea: 11% vs. 5%, abdominal pain: 8% vs. 3%, and nausea: 8% vs. 6% for Naldemedine and placebo, respectively).
Opioid Withdrawal
- In Studies 1, 2 and 3, adverse reactions consistent with opioid withdrawal were based on investigator assessment and adjudicated based upon the occurrence of at least 3 adverse reactions potentially related to opioid withdrawal with onset of a constellation of those symptoms occurring on the same day or within one day of each other.
- Adverse reactions of possible opioid withdrawal could include non-gastrointestinal (GI) symptoms (e.g., hyperhidrosis, hot flush or flushing, chills, tremor, tachycardia, anxiety, agitation, yawning, rhinorrhea, increased lacrimation, sneezing, feeling cold, and pyrexia), GI symptoms (e.g., vomiting, diarrhea, or abdominal pain), or both GI and non-GI symptoms.
- In pooled Studies 1 and 2, the incidence of adverse reactions of opioid withdrawal was 1% (8/542) for Naldemedine and 1% (3/546) for placebo. In Study 3 (52-week data), the incidence was 3% (20/621) for Naldemedine and 1% (9/619) for placebo. Most Naldemedine treated subjects experienced nearly equal incidence of GI only or both GI and non-GI symptoms.
Less Common Adverse Reactions:
- Two patients developed symptoms of hypersensitivity following a single dose of Naldemedine. One patient reported bronchospasm and another rash.
Postmarketing Experience
There is limited information regarding Naldemedine Postmarketing Experience in the drug label.
Drug Interactions
- Table 3 includes drugs with clinically important drug interactions with Naldemedine and instructions for preventing or managing the interaction.

Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data with Naldemedine in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. There is a potential for opioid withdrawal in a fetus when Naldemedine is used in pregnant women. Naldemedine should be used during pregnancy only if the potential benefit justifies the potential risk.
- In a rat embryo-fetal development study following oral administration of Naldemedine during the period of organogenesis at doses resulting in systemic exposure approximately 23,000 times the human area under the plasma-concentration time curve (AUC) at the recommended human dose of 0.2 mg/day, no developmental abnormalities were observed. In rabbits, there were no adverse effects on embryo-fetal development following oral administration of Naldemedine during the period of organogenesis at doses resulting in systemic exposure approximately 226 times the human AUC at the recommended human dose of 0.2 mg/day. No effects on pre- and postnatal development were observed in rats at exposures 12 times human exposures at the recommended human dose.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
- Naldemedine crosses the placenta, and may precipitate opioid withdrawal in a fetus due to the immature fetal blood-brain barrier.
Data (Animal)
- In rats, there were no adverse effects on embryo-fetal development following oral administration of Naldemedine during the period of organogenesis at doses up to 1000 mg/kg/day (approximately 23,000 times the human exposures (AUC) at the recommended human dose). In rabbits, there were no adverse effects on embryo-fetal development following oral administration of Naldemedine during the period of organogenesis at doses up to 100 mg/kg/day (approximately 226 times the human exposures (AUC) at the recommended human dose). At 400 mg/kg/day (approximately 844 times the human exposures (AUC) at the recommended human dose), effects in maternal animals included body weight loss/decreased body weight gain and food consumption, fetal loss, and premature delivery. Decreased fetal body weights at this dose may be related to the maternal toxicity observed.
- In the pre- and postnatal development study, pregnant rats were administered Naldemedine at oral doses up to 1000 mg/kg/day from gestation day 7 through lactation day 20. No effects on pre- and postnatal development were observed in rats at 1 mg/kg/day (approximately 12 times the human exposures (AUC) at the recommended human dose). A single dam died at parturition at 1000 mg/kg/day, and decreased body weights/body weight gain and food consumption, poor nursing, and total litter loss were noted at 30 and 1000 mg/kg/day (approximately 626 and 17,000 times the human exposures (AUC) at the recommended human dose, respectively). Decreases in the offspring viability index on Day 4 after birth were noted at 30 and 1000 mg/kg/day, and low body weights and delayed pinna unfolding in pups were noted at 1000 mg/kg/day.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Naldemedine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Naldemedine during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of Naldemedine in human milk, the effects on the breastfed infant, or the effects on milk production. Naldemedine was present in the milk of rats. Because of the potential for serious adverse reactions, including opioid withdrawal in breastfed infants, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother. If drug is discontinued in order to minimize drug exposure to a breastfed infant, advise women that breastfeeding may be resumed 3 days after the final dose of Naldemedine.
Data
- Drug-related radioactivity was transferred into milk of lactating rats following a single oral dose of 1 mg/kg [carbonyl-14C]-Naldemedine.
Pediatric Use
- The safety and effectiveness of Naldemedine have not been established in pediatric patients.
Geriatic Use
- Of 1163 patients in clinical studies exposed to Naldemedine, 183 (16%) were 65 years of age and over, while 37 (3%) were 75 years and over. No overall differences in safety or effectiveness between these and younger patients were observed, but greater sensitivity of some older individuals cannot be ruled out. In a population pharmacokinetic analysis, no age-related alterations in the pharmacokinetics of Naldemedine were observed.
Gender
There is no FDA guidance on the use of Naldemedine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Naldemedine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Naldemedine in patients with renal impairment.
Hepatic Impairment
- The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of Naldemedine has not been evaluated. Avoid use of Naldemedine in patients with severe hepatic impairment. No dose adjustment of Naldemedine is required in patients with mild or moderate hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Naldemedine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Naldemedine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Alteration of analgesic dosing regimen prior to initiating Naldemedine is not required.
- Patients receiving opioids for less than 4 weeks may be less responsive to Naldemedine.
- Discontinue Naldemedine if treatment with the opioid pain medication is also discontinued.
Monitoring
- Increased frequency of spontaneous bowel movements is indicative of efficacy.
- Development of severe, persistent, or worsening abdominal pain.
- Symptoms of opioid withdrawal.
IV Compatibility
There is limited information regarding the compatibility of Naldemedine and IV administrations.
Overdosage
- Single doses of Naldemedine up to 100 mg (500 times the recommended dose) and multiple doses of up to 30 mg (150 times the recommended dose) for 10 days have been administered to healthy subjects in clinical studies. Dose-dependent increases in gastrointestinal-related adverse reactions, including abdominal pain, diarrhea, and nausea, were observed.
- Single doses of Naldemedine up to 3 mg (15 times the recommended dose) and multiple doses of 0.4 mg (twice the recommended dose) for 28 days have been administered to patients with OIC in clinical studies. Dose-dependent increases in gastrointestinal-related adverse reactions, including abdominal pain, diarrhea, nausea, and vomiting, were observed. Also, chills, hyperhidrosis, and dizziness were reported more frequently at 1 and 3 mg doses and hyperhidrosis at the 0.4 mg dose.
- No antidote for Naldemedine is known. Hemodialysis is not an effective means to remove Naldemedine from the blood.
Pharmacology
| |
Naldemedine
| |
| Systematic (IUPAC) name | |
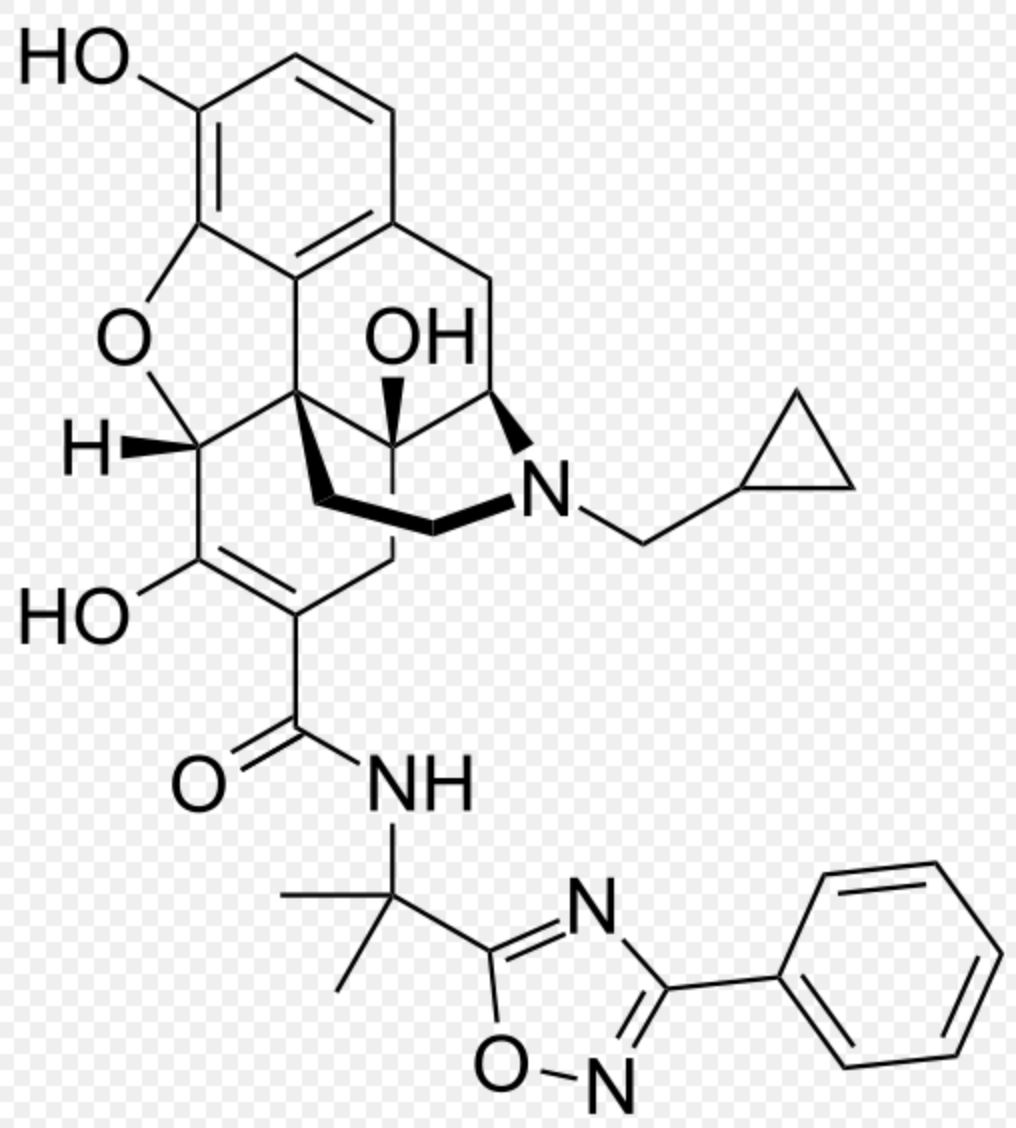
| 17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3,6,14-trihydroxy-N-[2-(3-phenyl-1,2,4-oxadiazol-5-yl)propan-2-yl]morphinan-7-carboxamide | |
| Identifiers | |
| CAS number | 1345728-04-2 (tosylate) |
| ATC code | None |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 570.63556 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
?(US) |
| Routes | Oral |
Mechanism of Action
- Naldemedine is an opioid antagonist with binding affinities for mu-, delta-, and kappa-opioid receptors. Naldemedine functions as a peripherally-acting mu-opioid receptor antagonist in tissues such as the gastrointestinal tract, thereby decreasing the constipating effects of opioids.
- Naldemedine is a derivative of naltrexone to which a side chain has been added that increases the molecular weight and the polar surface area, thereby reducing its ability to cross the blood-brain barrier (BBB).
- Naldemedine is also a substrate of the P-glycoprotein (P-gp) efflux transporter. Based on these properties, the CNS penetration of Naldemedine is expected to be negligible at the recommended dose levels, limiting the potential for interference with centrally-mediated opioid analgesia.
Structure

Pharmacodynamics
- Use of opioids induces slowing of gastrointestinal motility and transit. Antagonism of gastrointestinal mu-opioid receptors by Naldemedine inhibits opioid-induced delay of gastrointestinal transit time.
Effect on Cardiac Repolarization
- At a dose up to 5-times the recommended dose, Naldemedine does not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
Absorption
- Following oral administration, Naldemedine is absorbed with the time to achieve peak concentrations (Tmax) of approximately 0.75 hours in a fasted state. Across the range of doses evaluated, the maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) increased in a dose-proportional or almost dose-proportional manner. Accumulation was minimal following multiple daily doses of Naldemedine.
Food Effect
- A high-fat meal decreased the rate, but not the extent of Naldemedine absorption. The Cmax was decreased by approximately 35% and time to achieve Cmax was delayed from 0.75 hours in the fasted state to 2.5 hours in the fed state, whereas there was no meaningful change in the AUC in the fed state.
Distribution
- Plasma protein binding of Naldemedine in humans is 93% to 94%. The mean apparent volume of distribution during the terminal phase (Vz/F) is 155 L.
Elimination
- The terminal elimination half-life of Naldemedine is 11 hours.
Metabolism
- Naldemedine is primarily metabolized by CYP3A to nor-Naldemedine, with minor contribution from UGT1A3 to form Naldemedine 3-G. Nor-Naldemedine and Naldemedine 3-G have been shown to have antagonistic activity for opioid receptors, with less potent effect than Naldemedine.
- Following oral administration of [14C]-labeled Naldemedine, the primary metabolite in plasma was nor-Naldemedine, with a relative exposure compared to Naldemedine of approximately 9% to 13%. Naldemedine 3-G was a minor metabolite in plasma, with a relative exposure to Naldemedine of less than 3%.
- Naldemedine also undergoes cleavage in the GI tract to form benzamidine and Naldemedine carboxylic acid.
Excretion
- Following oral administration of [14C]-labeled Naldemedine, the total amount of radioactivity excreted in the urine and feces was 57% and 35% of the administered dose of Naldemedine, respectively. The amount of Naldemedine excreted unchanged in the urine was approximately 16% to 18% of the administered dose. Benzamidine was the most predominant metabolite excreted in the urine and feces, representing approximately 32% and 20% of the administered dose of Naldemedine, respectively. The percentage of unchanged drug in feces has not been estimated.
Use in Specific Populations
Age: Geriatric Population, Sex, Race/Ethnicity
- A population pharmacokinetic analysis from clinical studies with Naldemedine did not identify a clinically meaningful effect of age, sex, or race on the pharmacokinetics of Naldemedine.
Renal Impairment
- The pharmacokinetics of Naldemedine after administration of a 0.2 mg single oral dose of Naldemedine was studied in 8 subjects with mild (n=8, estimated glomerular filtration rate [eGFR] of 60 to 89 mL/min/1.73 m2), moderate (n=8, eGFR 30 to 59 mL/min/1.73 m2), and severe (n=6, eGFR less than 30 mL/min/1.73 m2) renal impairment, and subjects with end-stage renal disease (ESRD) requiring hemodialysis (n=8), and compared to healthy subjects with normal renal function (n=8, estimated creatinine clearance of at least 90 mL/min). The pharmacokinetics of Naldemedine between subjects in all groups were similar.
- Plasma concentrations of Naldemedine in subjects with ESRD requiring hemodialysis were similar when Naldemedine was administered either pre- or post-hemodialysis, indicating that Naldemedine was not removed from the blood by hemodialysis.
Hepatic Impairment
- The effect of hepatic impairment on the pharmacokinetics of a 0.2 mg single oral dose of Naldemedine was studied in subjects with hepatic impairment classified as mild (n=8, Child-Pugh Class A) or moderate (n=8, Child-Pugh Class B) and compared with healthy subjects with normal hepatic function (n=8). The pharmacokinetics of Naldemedine between subjects in all groups were similar.
- The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of Naldemedine was not evaluated.
Drug Interaction Studies
Effect of Naldemedine on Other Drugs
- In in vitro studies at clinically relevant concentrations, Naldemedine did not inhibit the major CYP enzymes (including CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or CYP4A11 isozymes) and is not an inhibitor of transporters (including OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, BCRP, or P-gp). Naldemedine did not cause significant induction of CYP1A2, CYP2B6, CYP3A4, UGT1A2, UGT1A6, or UGT2B7 isozymes.
Effect of Other Drugs on Naldemedine
- Naldemedine is primarily metabolized by CYP3A4 enzyme with minor contribution from UGT1A3. Naldemedine is a substrate of P-gp. The effects of co-administered drugs on the pharmacokinetics of Naldemedine are summarized in Figure 1.

Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- In 2-year carcinogenicity studies, there were no drug-related neoplastic findings following oral administration of Naldemedine to mice and rats at doses up to 100 mg/kg/day (approximately 17,500 and 6,300 times the human exposures (AUC) at the recommended human dose, respectively).
Mutagenesis
- Naldemedine was not genotoxic in the in vitro bacterial reverse mutation (Ames) assay, a chromosomal aberration assay with cultured Chinese hamster lung cells, and an in vivo micronucleus assay with rat bone marrow cells.
Impairment of Fertility
- Naldemedine was found to have no effect on fertility or reproductive performance in male and female rats at oral doses up to 1000 mg/kg/day (approximately 17,000 times the human exposures (AUC) at the recommended human dose). In female rats, prolongation of diestrous phase was noted at 10 mg/kg/day (approximately 179 times the human exposures (AUC) at the recommended human dose).
Clinical Studies
- Naldemedine was evaluated in two replicate, 12-week, randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) in which Naldemedine was used without laxatives in patients with OIC and chronic non-cancer pain.
- Patients receiving a stable opioid morphine equivalent daily dose of at least 30 mg for at least 4 weeks before enrollment and self-reported OIC were eligible for clinical trial participation.
- Patients with evidence of significant structural abnormalities of the GI tract were not enrolled in these trials.
- In Studies 1 and 2, patients had to either be not using laxatives or willing to discontinue laxative use at the time of screening and willing to use only the provided rescue laxatives during the screening and treatment periods.
- In Studies 1 and 2, OIC was confirmed through a two-week run in period and was defined as no more than 4 spontaneous bowel movements (SBMs) total over 14 consecutive days and less than 3 SBMs in a given week with at least 25% of the SBMs associated with one or more of the following conditions: (1) straining; (2) hard or lumpy stools; (3) having a sensation of incomplete evacuation; and (4) having a sensation of anorectal obstruction/blockage.
- An SBM was defined as a bowel movement (BM) without rescue laxative taken within the past 24 hours. Patients with no BMs over the 7 consecutive days prior to and during the 2 week screening period or patients who have never taken laxatives were excluded.
- In the screening and treatment periods, bisacodyl was used as rescue laxative if patients had not had a BM for 72 hours and were allowed one-time use of an enema, if after 24 hours of taking bisacodyl they still had not had a BM.
- A total of 547 patients in Study 1 and 553 patients in Study 2 were randomized in a 1:1 ratio to receive Naldemedine 0.2 mg once daily or placebo for 12 weeks. Study medication was administered without regard to meals.
- The mean age of subjects in Studies 1 and 2 was 54 years; 59% were women; and 80% were white. The most common types of pain in Studies 1 and 2 were back or neck pain (61%). The mean baseline number of SBMs was 1.3 and 1.2 per week for Studies 1 and 2, respectively.
- Prior to enrollment, patients were using their current opioid for a mean duration of approximately 5 years. A wide range of types of opioids were used. The mean baseline opioid morphine equivalent daily dosage was 132 mg and 121 mg per day for Studies 1 and 2, respectively.
- The efficacy of Naldemedine was assessed in Studies 1 and 2 using a responder analysis. A responder was defined as a patient who had at least 3 SBMs per week and a change from baseline of at least 1 SBM per week for at least 9 out of the 12 weeks and 3 out of the last 4 weeks in Studies 1 and 2.
- The responder rates in Studies 1 and 2 are shown in Table 4.

- In Studies 1 and 2, the mean increase in frequency of SBMs per week from baseline to the last 2 weeks of the 12-week treatment period was 3.1 for Naldemedine vs. 2.0 for placebo (difference 1.0, 95% CI 0.6, 1.5), and 3.3 for Naldemedine vs. 2.1 for placebo (difference 1.2, 95% CI 0.8, 1.7), respectively.
- During week 1 of the treatment period, the mean increase in frequency of SBMs per week from baseline was 3.3 for Naldemedine vs. 1.3 for placebo (difference 2.0, 95% CI 1.5, 2.5) in Study 1 and 3.7 for Naldemedine vs. 1.6 for placebo (difference 2.1, 95% CI 1.5, 2.6) in Study 2.
- The mean increase in the frequency of complete SBM (CSBM) per week from baseline to the last 2 weeks of 12-week treatment period was 2.3 for Naldemedine vs. 1.5 for placebo (difference 0.8, 95% CI 0.4, 1.2) in Study 1 and 2.6 for Naldemedine vs. 1.6 for placebo (difference 1.1, 95% CI 0.6, 1.5) in Study 2. A CSBM was defined as a SBM that was associated with a sense of complete evacuation.
- The change in the frequency of SBMs without straining per week from baseline to the last 2 weeks of the treatment period was 1.3 for Naldemedine vs. 0.7 for placebo (difference 0.6, 95% CI 0.2, 0.9) in Study 1 and 1.8 for Naldemedine vs. 1.1 for placebo (difference 0.7, 95% CI 0.3, 1.2) in Study 2.
How Supplied
- Naldemedine is supplied as 0.2 mg Naldemedine tablets in:
- bottle of 30 tablets - NDC 59011-523-30
- bottle of 90 tablets - NDC 59011-523-90
Storage
- Store Naldemedine in light resistant container at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Naldemedine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Naldemedine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Administration
- Advise patients to discontinue Naldemedine if treatment with the opioid pain medication is also discontinued.
Gastrointestinal Perforation
- Advise patients to discontinue Naldemedine and to promptly seek medical attention if they develop unusually severe, persistent or worsening abdominal pain.
Opioid Withdrawal
- Advise patients that clusters of symptoms consistent with opioid withdrawal may occur while taking Naldemedine and to contact their healthcare provider if these symptoms occur.
Pregnancy
- Advise females of reproductive potential, who become pregnant or are planning to become pregnant, that the use of Naldemedine during pregnancy may precipitate opioid withdrawal in a fetus due to the undeveloped blood-brain barrier.
Lactation
- Advise women that breastfeeding is not recommended during treatment with Naldemedine and for 3 days after the final dose.

Precautions with Alcohol
Alcohol-Naldemedine interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Symproic
Look-Alike Drug Names
There is limited information regarding Naldemedine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.