Nadolol (tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal: Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered nadolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of one to two weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, nadolol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue nadolol therapy abruptly even in patients treated only for hypertension.
|
Overview
Nadolol (tablet) is a beta-adrenergic blocker that is FDA approved for the {{{indicationType}}} of agina pectoris, hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bradyarrhythmia, dizziness, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Angina Pectoris
- Dosing infromation
- The usual initial dose is 40 mg nadolol once daily. Dosage may be gradually increased in 40 to 80 mg increments at 3 to 7 day intervals until optimum clinical response is obtained or there is pronounced slowing of the heart rate. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 160 or 240 mg administered once daily may be needed.
- The usefulness and safety in angina pectoris of dosage exceeding 240 mg per day have not been established. If treatment is to be discontinued, reduce the dosage gradually over a period of one to two weeks
Hypertension
- Dosing infromation
- The usual initial dose is 40 mg nadolol once daily, whether it is used alone or in addition to diuretic therapy. Dosage may be gradually increased in 40 to 80 mg increments until optimum blood pressure reduction is achieved. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 240 or 320 mg administered once daily may be needed.
Dosage Adjustment in Renal Failure
Absorbed nadolol is excreted principally by the kidneys and, although nonrenal elimination does occur, dosage adjustments are necessary in patients with renal impairment. The following dose intervals are recommended:
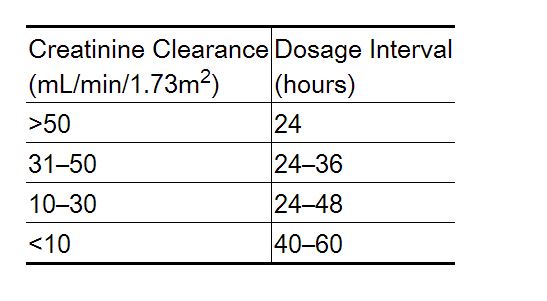
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Atrial Fibrillation, Heart Rate Control
- Developed by: AHA/ACC
- Class of Recommendation: Class I
- Strength of Evidence: Level B
- Dosing Information/Recommendation
- 10 to 240 mg PO daily[1]
Non–Guideline-Supported Use
Supraventricular Tachycardia
- Dosing information
- Initial dose of 0.01 followed by 0.02 and then 0.04 mg/kg administered every 10 minutes until achieving a positive response or the maximum dose of 0.07 mg/kg is reached. Do not exceed 10 mg.[2]
Gastrointestinal Hemorrhage
- Dosing Information
- 40 to 160 mg/day[3]
Hyperthyroidism
- Dosing Information
- 80 to 160 mg/day[4]
Migraine Prophylaxis
- Dosing Information
Tremor
- Dosing information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Nadolol (tablet) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nadolol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nadolol in pediatric patients.
Contraindications
- Bronchial asthma
- Sinus bradycardia
- Second degree conduction block
- Third degree conduction block
- Cardiogenic shock
- Cardiac failure
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal: Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered nadolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of one to two weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, nadolol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue nadolol therapy abruptly even in patients treated only for hypertension.
|
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Nadolol (tablet)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Nadolol (tablet) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nadolol (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nadolol (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Nadolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nadolol (tablet) Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE; et al. (2014). "2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". J Am Coll Cardiol. doi:10.1016/j.jacc.2014.03.022. PMID 24685669.
- ↑ Olukotun AY, Klein GJ (1987). "Efficacy and safety of intravenous nadolol for supraventricular tachycardia". Am J Cardiol. 60 (6): 59D–62D. PMID 3630923.
- ↑ Gatta A, Merkel C, Sacerdoti D, Bolognesi M, Caregaro L, Zuin R; et al. (1987). "Nadolol for prevention of variceal rebleeding in cirrhosis: a controlled clinical trial". Digestion. 37 (1): 22–8. PMID 3301478.
- ↑ Lazarus JH, Kingswood JC, John R (1987). "The effect of nadolol on heart rate in hyperthyroidism. A controlled trial". Acta Endocrinol (Copenh). 114 (1): 102–6. PMID 3544631.
- ↑ Pascual J, Rivas MT, Leira R (2007). "Testing the combination beta-blocker plus topiramate in refractory migraine". Acta Neurol Scand. 115 (2): 81–3. doi:10.1111/j.1600-0404.2006.00772.x. PMID 17212609.
- ↑ Ryan RE, Ryan RE, Sudilovsky A (1983). "Nadolol: its use in the prophylactic treatment of migraine". Headache. 23 (1): 26–31. PMID 6131052.
- ↑ Edwards RV (1982). "Nadolol use for cerebellar tremor". Am J Psychiatry. 139 (11): 1522. PMID 6127958.
- ↑ Koller WC (1983). "Nadolol in essential tremor". Neurology. 33 (8): 1076–7. PMID 6348587.