Mycophenolate sodium
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, AND SERIOUS INFECTIONS
See full prescribing information for complete Boxed Warning.
* Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations.
|
Overview
Mycophenolate sodium is an immunosuppressant that is FDA approved for the prophylaxis of organ rejection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include anemia, leukopenia, constipation, nausea, diarrhea, vomiting, dyspepsia, urinary tract infection, CMV infection, insomnia, and postoperative pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Mycophenolate sodium FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mycophenolic acid in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mycophenolic acid in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Mycophenolate sodium FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mycophenolic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mycophenolic acid in pediatric patients.
Contraindications
Hypersensitivity Reactions
Mycophenolic acid delayed-release tablets are contraindicated in patients with a hypersensitivity to mycophenolate sodium, mycophenolic acid, mycophenolate mofetil, or to any of its excipients. Reactions like rash, pruritus, hypotension and chest pain have been observed in clinical trials and post marketing reports
Warnings
|
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, AND SERIOUS INFECTIONS
See full prescribing information for complete Boxed Warning.
* Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations.
|
Embryofetal Toxicity
Use of mycophenolic acid delayed-release tablets during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, and kidney [see Use in Specific Populations (8.1)].
Pregnancy Exposure Prevention and Planning
Females of reproductive potential must be aware of the increased risk of first trimester pregnancy loss and congenital malformations and must be counseled regarding pregnancy prevention and planning. For recommended pregnancy testing and contraception methods.
Management of Immunosuppression
Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physicians responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
Lymphoma and Other Malignancies
Patients receiving immunosuppressants, including mycophenolic acid delayed-release tablets, are at increased risk of developing lymphomas and other malignancies, particularly of the skin [see Adverse Reactions (6)]. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
As usual for patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
Post-transplant lymphoproliferative disorder (PTLD) has been reported in immunosuppressed organ transplant recipients. The majority of PTLD events appear related to Epstein Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children.
Serious Infections
Patients receiving immunosuppressants, including mycophenolic acid delayed-release tablets, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, and new or reactivated viral infections including opportunistic infections [see Warnings and Precautions (5.6)]. These infections may lead to serious, including fatal outcomes. Because of the danger of oversuppression of the immune system which can increase susceptibility to infection, combination immunosuppressant therapy should be used with caution.
New or Reactivated Viral Infections
Polyomavirus associated nephropathy (PVAN), JC virus associated progressive multifocal leukoencephalopathy (PML), cytomegalovirus (CMV) infections, reactivation of hepatitis B (HBV) or hepatitis C (HCV) have been reported in patients treated with immunosuppressants, including the mycophenolic acid (MPA) derivatives mycophenolic acid delayed-release tablets and MMF. Reduction in immunosuppression should be considered for patients who develop evidence of new or reactivated viral infections. Physicians should also consider the risk that reduced immunosuppression represents to the functioning allograft.
PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss. Patient monitoring may help detect patients at risk for PVAN.
PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated.
The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease.
Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
Blood Dyscrasias Including Pure Red Cell Aplasia
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with MPA derivatives in combination with other immunosuppressive agents. The mechanism for MPA derivatives induced PRCA is unknown; the relative contribution of other immunosuppressants and their combinations in an immunosuppressive regimen is also unknown. In some cases PRCA was found to be reversible with dose reduction or cessation of therapy with MPA derivatives. In transplant patients, however, reduced immunosuppression may place the graft at risk. Changes to mycophenolic acid therapy should only be undertaken under appropriate supervision in transplant recipients in order to minimize the risk of graft rejection.
Patients receiving mycophenolic acid should be monitored for blood dyscrasias (e.g., neutropenia or anemia). The development of neutropenia may be related to mycophenolic acid itself, concomitant medications, viral infections, or some combination of these reactions. Complete blood count should be performed weekly during the first month, twice monthly for the second and the third month of treatment, then monthly through the first year. If blood dyscrasias occur [neutropenia develops (ANC <1.3×103/mcL) or anemia], dosing with mycophenolic acid should be interrupted or the dose reduced, appropriate tests performed, and the patient managed accordingly.
Serious GI Tract Complications
Gastrointestinal bleeding (requiring hospitalization), intestinal perforations, gastric ulcers, and duodenal ulcers have been reported in patients treated with mycophenolic acid delayed-release tablets. Mycophenolic acid delayed-release tablets should be administered with caution in patients with active serious digestive system disease.
Immunizations
The use of live attenuated vaccines should be avoided during treatment with mycophenolic acid delayed-release tablets; examples include (but not limited to) the following: intranasal influenza, measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid vaccines.
Rare Hereditary Deficiencies
Mycophenolic acid is an inosine monophosphate dehydrogenase inhibitor (IMPDH Inhibitor). Mycophenolic acid should be avoided in patients with rare hereditary deficiency of hypoxanthine-guanine phosphoribosyl-transferase (HGPRT) such as Lesch- Nyhan and Kelley-Seegmiller syndromes because it may cause an exacerbation of disease symptoms characterized by the overproduction and accumulation of uric acid leading to symptoms associated with gout such as acute arthritis, tophi, nephrolithiasis or urolithiasis and renal disease including renal failure.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are discussed in greater detail in other sections of the label.
- Embryofetal Toxicity
- Lymphomas and Other Malignancies
- Serious Infections
- New or Reactivated Viral Infections
- Blood Dyscrasias Including Pure Red Cell Aplasia
- Serious GI Tract Complications
- Rare Hereditary Deficiencies
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below derive from two randomized, comparative, active-controlled, double-blind, double-dummy trials in prevention of acute rejection in de novo and converted stable kidney transplant patients.
- In the de novo trial, patients were administered either mycophenolic acid delayed-release tablets 1.44 grams per day (N=213) or MMF 2 grams per day (N=210) within 48 hours post-transplant for 12 months in combination with cyclosporine, USP MODIFIED and corticosteroids. Forty-one percent of patients also received antibody therapy as induction treatment. In the conversion trial, renal transplant patients who were at least 6 months post-transplant and receiving 2 grams per day MMF in combination with cyclosporine USP MODIFIED, with or without corticosteroids for at least two weeks prior to entry in the trial were randomized to mycophenolic acid delayed-release tablets 1.44 grams per day (N=159) or MMF 2 grams per day (N=163) for 12 months.
- The average age of patients in both studies was 47 years and 48 years (de novo study and conversion study, respectively), ranging from 22 to 75 years. Approximately 66% of patients were male; 82% were white, 12 % were black, and 6% other races. About 40% of patients were from the United States and 60% from other countries.
- In the de novo trial, the overall incidence of discontinuation due to adverse reactions was 18% (39/213) and 17% (35/210) in the mycophenolic acid delayed-release tablets and MMF arms, respectively. The most common adverse reactions leading to discontinuation in the mycophenolic acid arm were graft loss (2%), diarrhea (2%), vomiting (1%), renal impairment (1%), CMV infection (1%), and leukopenia (1%). The overall incidence of patients reporting dose reduction at least once during the 0 to12 month study period was 59% and 60% in the mycophenolic acid delayed-release tablets and MMF arms, respectively. The most frequent reasons for dose reduction in the mycophenolic acid delayed-release tablets arm were adverse reactions (44%), dose reductions according to protocol guidelines (17%), dosing errors (11%) and missing data (2%).
- The most common adverse reactions (≥20%) associated with the administration of mycophenolic acid delayed-release tablets were anemia, leukopenia, constipation, nausea, diarrhea, vomiting, dyspepsia, urinary tract infection, CMV infection, insomnia and postoperative pain.
- The adverse reactions reported in ≥10% of patients in the de novo trial are presented in Table 2 below.
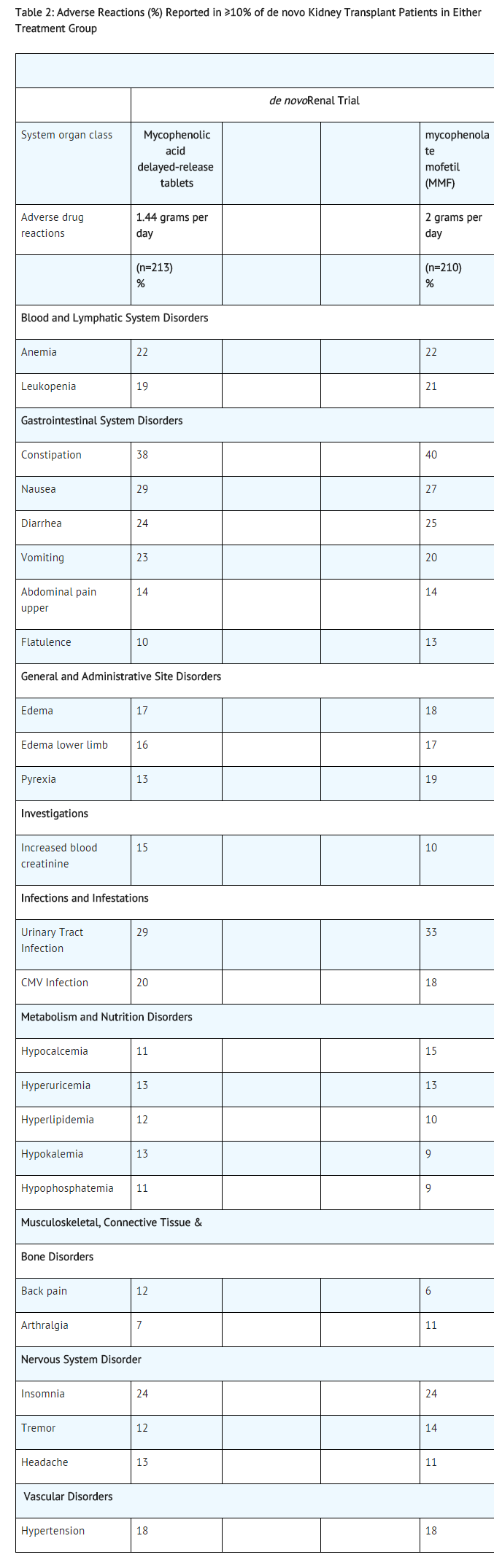
- The trial was not designed to support comparative claims for mycophenolic acid for the adverse reactions reported in this table.
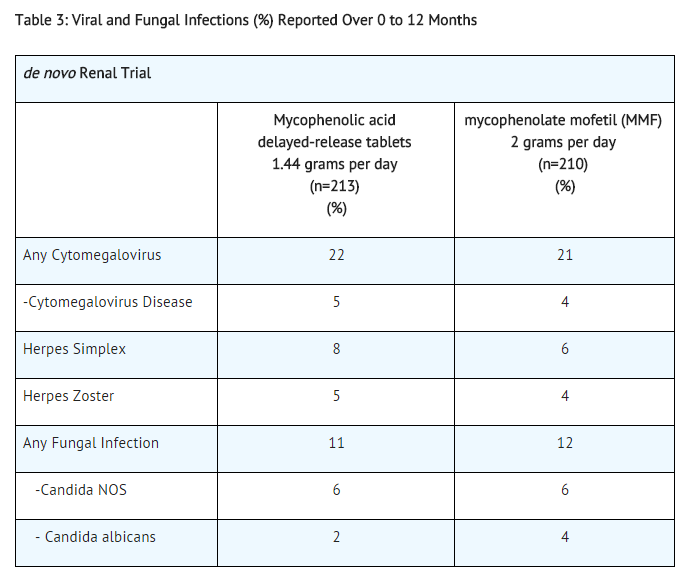
Table 3 summarizes the incidence of opportunistic infections in de novo transplant patients.
Lymphoma developed in 2 de novo patients (1%), (1 diagnosed 9 days after treatment initiation) and in 2 conversion patients (1%) receiving mycophenolic acid delayed-release tablets with other immunosuppressive agents in the 12-month controlled clinical trials.
Non melanoma skin carcinoma occurred in 1% de novo and 12% conversion patients. Other types of malignancy occurred in 1% de novo and 1% conversion patients.
The adverse reactions reported in <10% of de novo or conversion patients treated with mycophenolic acid delayed-release tablets in combination with cyclosporine and corticosteroids are listed in Table 4.
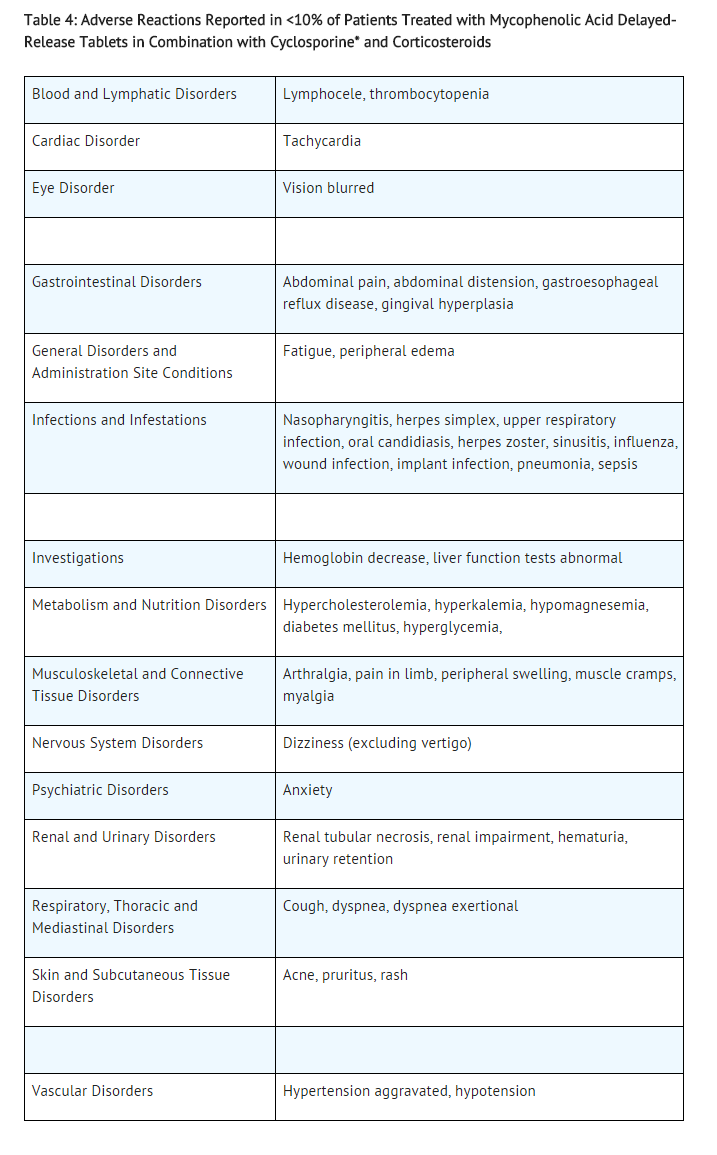
The following additional adverse reactions have been associated with the exposure to mycophenolic acid (MPA) when administered as a sodium salt or as mofetil ester:
Gastrointestinal: Intestinal perforation, gastrointestinal hemorrhage, gastric ulcers, duodenal ulcers, colitis (including CMV colitis), pancreatitis, esophagitis, and ileus.
Infections: Serious life-threatening infections such as meningitis and infectious endocarditis, tuberculosis, and atypical mycobacterial infection.
Respiratory: Interstitial lung disorders, including fatal pulmonary fibrosis.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of mycophenolic acid or other MPA derivatives. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Congenital malformations and an increased incidence of first trimester pregnancy loss have been reported following exposure to MMF during pregnancy.
- Infections
Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal.
- Polyomavirus associated nephropathy (PVAN), especially due to BK virus infection, associated with serious outcomes, including deteriorating renal function and renal graft loss.
- Viral reactivation in patients infected with HBV or HCV.
- Cases of pure red cell aplasia (PRCA) have been reported in patients treated with MPA derivatives in combination with other immunosuppressive agents .
The following additional adverse reactions have been identified during postapproval use of mycophenolic acid: agranulocytosis, asthenia, osteomyelitis, lymphadenopathy, lymphopenia, wheezing, dry mouth, gastritis, peritonitis, anorexia, alopecia, pulmonary edema, Kaposi’s sarcoma.
Drug Interactions
Antacids with Magnesium and Aluminum Hydroxides
Concomitant use of mycophenolic acid delayed-release tablets and antacids decreased plasma concentrations of mycophenolic acid (MPA). It is recommended that mycophenolic acid delayed-release tablets and antacids not be administered simultaneously [see Clinical Pharmacology (12.3)].
Azathioprine
Given that azathioprine and MMF inhibit purine metabolism, it is recommended that mycophenolic acid delayed-release tablets not be administered concomitantly with azathioprine or MMF.
Cholestyramine, Bile Acid Sequestrates, Oral Activated Charcoal and Other Drugs that Interfere with Enterohepatic Recirculation
Drugs that interrupt enterohepatic recirculation may decrease MPA plasma concentrations when coadministered with MMF. Therefore, do not administer mycophenolic acid delayed-release tablets with cholestyramine or other agents that may interfere with enterohepatic recirculation or drugs that may bind bile acids, e.g., bile acid sequestrates or oral activated charcoal, because of the potential to reduce the efficacy of mycophenolic acid delayed-release tablets.
Sevelamer
Concomitant administration of sevelamer and MMF may decrease MPA plasma concentrations. Sevelamer and other calcium free phosphate binders should not be administered simultaneously with mycophenolic acid delayed-release tablets.
Cyclosporine
Cyclosporine inhibits the enterohepatic recirculation of MPA, and therefore, MPA plasma concentrations may be decreased when mycophenolic acid delayed-release tablets are coadministered with cyclosporine. Clinicians should be aware that there is also a potential change of MPA plasma concentrations after switching from cyclosporine to other immunosuppressive drugs or from other immunosuppressive drugs to cyclosporine in patients concomitantly receiving mycophenolic acid delayed-release tablets.
Norfloxacin and Metronidazole
MPA plasma concentrations may be decreased when MMF is administrated with norfloxacin and metronidazole. Therefore, mycophenolic acid delayed-release tablets are not recommended to be given with the combination of norfloxacin and metronidazole. Although there will be no effect on MPA plasma concentrations when mycophenolic acid delayed-release tablets are concomitantly administered with norfloxacin or metronidazole when given separately [see Clinical Pharmacology (12.3)].
Rifampin
The concomitant administration of MMF and rifampin may decrease MPA plasma concentrations. Therefore, mycophenolic acid delayed-release tablets are not recommended to be given with rifampin concomitantly unless the benefit outweighs the risk.
Hormonal Contraceptives
In a drug interaction study, mean levonorgestrel AUC was decreased by 15% when coadministered with MMF. Although mycophenolic acid delayed-release tablets may not have any influence on the ovulation-suppressing action of oral contraceptives, it is recommended to coadminister mycophenolic acid delayed- release tablets with hormonal contraceptives (e.g., birth control pill, transdermal patch, vaginal ring, injection, and implant) with caution, and additional barrier contraceptive methods must be used.
Acyclovir (Valacyclovir), Ganciclovir (Valganciclovir), and Other Drugs that Undergo Renal Tubular Secretion
The coadministration of MMF and acyclovir or ganciclovir may increase plasma concentrations of mycophenolic acid glucuronide (MPAG) and acyclovir/valacyclovir/ganciclovir/valganciclovir as their coexistence competes for tubular secretion. Both acyclovir/valacyclovir/ganciclovir/valganciclovir and MPAG concentrations will be also increased in the presence of renal impairment. Acyclovir/valacyclovir/ganciclovir/valganciclovir may be taken with mycophenolic acid; however, during the period of treatment, physicians should monitor blood cell counts.
Ciprofloxacin, Amoxicillin plus Clavulanic Acid and Other Drugs that Alter the Gastrointestinal Flora
Drugs that alter the gastrointestinal flora such as ciprofloxacin or amoxicillin plus clavulanic acid may interact with MMF by disrupting enterohepatic recirculation. Interference of MPAG hydrolysis may lead to less MPA available for absorption when mycophenolic acid delayed-release tablet is concomitantly administered with ciprofloxacin or amoxicillin plus clavulanic acid. The clinical relevance of this interaction is unclear; however, no dose adjustment of mycophenolic acid delayed-release tablet is needed when coadministered with these drugs.
Pantoprazole
Administration of a pantoprazole at a dose of 40 mg twice daily for 4 days to healthy volunteers did not alter the pharmacokinetics of a single dose of mycophenolic acid delayed-release tablets.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Mycophenolate sodium in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mycophenolate sodium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mycophenolate sodium during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Mycophenolate sodium in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Mycophenolate sodium in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Mycophenolate sodium in geriatric settings.
Gender
There is no FDA guidance on the use of Mycophenolate sodium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mycophenolate sodium with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mycophenolate sodium in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mycophenolate sodium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mycophenolate sodium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mycophenolate sodium in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Mycophenolate sodium Administration in the drug label.
Monitoring
There is limited information regarding Mycophenolate sodium Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Mycophenolate sodium and IV administrations.
Overdosage
Signs and Symptoms
- There have been anecdotal reports of deliberate or accidental overdoses with mycophenolic acid delayed-release tablets, whereas not all patients experienced related adverse reactions.
- In those overdose cases in which adverse reactions were reported, the reactions fall within the known safety profile of the class. Accordingly an overdose of mycophenolic acid delayed-release tablets could possibly result in over suppression of the immune system and may increase the susceptibility to infection including opportunistic infections, fatal infections and sepsis. If blood dyscrasias occur (e.g., neutropenia with absolute neutrophil count <1.5 x 103/mcL or anemia), it may be appropriate to interrupt or discontinue mycophenolic acid delayed-release tablets.
- Possible signs and symptoms of acute overdose could include the following: hematological abnormalities such as leukopenia and neutropenia, and gastrointestinal symptoms such as abdominal pain, diarrhea, nausea and vomiting, and dyspepsia.
Treatment and Management
General supportive measures and symptomatic treatment should be followed in all cases of overdosage. Although dialysis may be used to remove the inactive metabolite mycophenolic acid glucuronide (MPAG), it would not be expected to remove clinically significant amounts of the active moiety, mycophenolic acid, due to the 98% plasma protein binding of mycophenolic acid. By interfering with enterohepatic circulation of mycophenolic acid, activated charcoal or bile sequestrates, such as cholestyramine, may reduce the systemic mycophenolic acid exposure.
Pharmacology
Mechanism of Action
There is limited information regarding Mycophenolate sodium Mechanism of Action in the drug label.
Structure
There is limited information regarding Mycophenolate sodium Structure in the drug label.
Pharmacodynamics
There is limited information regarding Mycophenolate sodium Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Mycophenolate sodium Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Mycophenolate sodium Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Mycophenolate sodium Clinical Studies in the drug label.
How Supplied
There is limited information regarding Mycophenolate sodium How Supplied in the drug label.
Storage
There is limited information regarding Mycophenolate sodium Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mycophenolate sodium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mycophenolate sodium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Mycophenolate sodium Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Mycophenolic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Mycophenolate sodium Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Mycophenolate sodium Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.