Mycophenolate sodium: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 7: | Line 7: | ||
|adverseReactions=[[anemia]], [[leukopenia]], [[constipation]], [[nausea]], [[diarrhea]], [[vomiting]], [[dyspepsia]], [[urinary tract infection]], [[CMV infection]], [[insomnia]], and [[postoperative pain]] | |adverseReactions=[[anemia]], [[leukopenia]], [[constipation]], [[nausea]], [[diarrhea]], [[vomiting]], [[dyspepsia]], [[urinary tract infection]], [[CMV infection]], [[insomnia]], and [[postoperative pain]] | ||
|blackBoxWarningTitle=WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, AND SERIOUS INFECTIONS | |blackBoxWarningTitle=WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, AND SERIOUS INFECTIONS | ||
|blackBoxWarningBody= | |blackBoxWarningBody=* Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. | ||
* Females of reproductive potential must be counseled regarding pregnancy prevention and planning, Use in Specific Populations. | |||
* Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression. | |||
* Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections [see Warnings and Precautions. | |||
* Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid. Patients receiving mycophenolic acid should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. | |||
|fdaLIADAdult===Indications== | |fdaLIADAdult===Indications== | ||
| Line 36: | Line 44: | ||
[[File:mycophenolic acid table01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:mycophenolic acid table01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Mycophenolic acid in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Mycophenolic acid in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Mycophenolic acid in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Mycophenolic acid in adult patients. | ||
Revision as of 17:42, 15 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, AND SERIOUS INFECTIONS
See full prescribing information for complete Boxed Warning.
* Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations.
|
Overview
Mycophenolate sodium is a {{{drugClass}}} that is FDA approved for the prophylaxis of organ rejection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include anemia, leukopenia, constipation, nausea, diarrhea, vomiting, dyspepsia, urinary tract infection, CMV infection, insomnia, and postoperative pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Prophylaxis of Organ Rejection in Kidney Transplant
- Mycophenolic acid is indicated for the prophylaxis of organ rejection in adult patients receiving a kidney transplant.
- Mycophenolic acid is indicated for the prophylaxis of organ rejection in pediatric patients 5 years of age and older who are at least 6 months post kidney transplant.
- Mycophenolic acid is to be used in combination with cyclosporine and corticosteroids.
Limitations of Use
Mycophenolic acid delayed-release tablets and mycophenolate mofetil (MMF) tablets and capsules should not be used interchangeably without physician supervision because the rate of absorption following the administration of these two products is not equivalent.
Dosage
Dosage in Adult Kidney Transplant Patients
The recommended dose of mycophenolic acid delayed-release tablets is 720 mg administered twice daily (1440 mg total daily dose).
Dosage in Pediatric Kidney Transplant Patients
The recommended dose of mycophenolic acid in conversion (at least 6 months post-transplant) pediatric patients age 5 years and older is 400 mg/m2 body surface area (BSA) administered twice daily (up to a maximum dose of 720 mg administered twice daily).
DOSAGE FORMS AND STRENGTHS
Mycophenolic acid delayed-release tablets are available as 180 mg and 360 mg tablets.
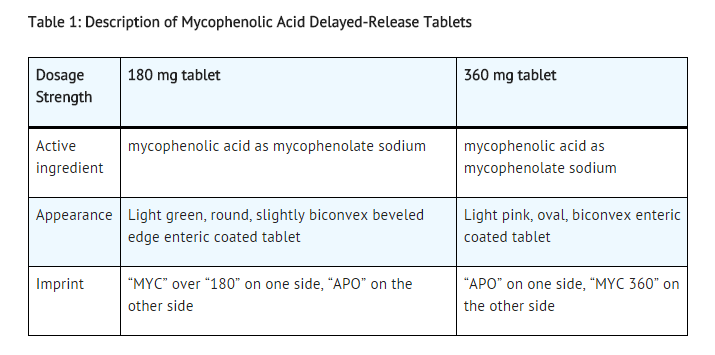
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mycophenolic acid in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mycophenolic acid in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Mycophenolate sodium FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mycophenolic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mycophenolic acid in pediatric patients.
Contraindications
There is limited information regarding Mycophenolate sodium Contraindications in the drug label.
Warnings
|
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, AND SERIOUS INFECTIONS
See full prescribing information for complete Boxed Warning.
* Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations.
|
There is limited information regarding Mycophenolate sodium Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Mycophenolate sodium Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Mycophenolate sodium Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Mycophenolate sodium Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Mycophenolate sodium in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mycophenolate sodium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mycophenolate sodium during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Mycophenolate sodium in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Mycophenolate sodium in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Mycophenolate sodium in geriatric settings.
Gender
There is no FDA guidance on the use of Mycophenolate sodium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mycophenolate sodium with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mycophenolate sodium in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mycophenolate sodium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mycophenolate sodium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mycophenolate sodium in patients who are immunocompromised.
Administration and Monitoring
Administration
- ORAL
- Mycophenolic acid delayed-release tablets should be taken on an empty stomach, 1 hour before or 2 hours after food intake.
- Mycophenolic acid delayed-release tablets should not be crushed, chewed, or cut prior to ingesting. The tablets should be swallowed whole in order to maintain the integrity of the enteric coating.
- Pediatric patients with a BSA of 1.19 to 1.58 m2 may be dosed either with three mycophenolic acid 180 mg tablets, or one 180 mg tablet plus one 360 mg tablet twice daily (1080 mg daily dose). Patients with a BSA of >1.58 m2 may be dosed either with four mycophenolic acid 180 mg tablets, or two mycophenolic acid 360 mg tablets twice daily (1440 mg daily dose). Pediatric doses for patients with BSA <1.19 m2 cannot be accurately administered using currently available formulations of mycophenolic acid tablets.
Monitoring
There is limited information regarding Mycophenolate sodium Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Mycophenolate sodium and IV administrations.
Overdosage
There is limited information regarding Mycophenolate sodium overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Mycophenolate sodium Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Mycophenolate sodium Mechanism of Action in the drug label.
Structure
There is limited information regarding Mycophenolate sodium Structure in the drug label.
Pharmacodynamics
There is limited information regarding Mycophenolate sodium Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Mycophenolate sodium Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Mycophenolate sodium Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Mycophenolate sodium Clinical Studies in the drug label.
How Supplied
There is limited information regarding Mycophenolate sodium How Supplied in the drug label.
Storage
There is limited information regarding Mycophenolate sodium Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mycophenolate sodium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mycophenolate sodium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Mycophenolate sodium Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Mycophenolic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Mycophenolate sodium Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Mycophenolate sodium Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.