Mirtazapine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|indication=major depressive disorder | |indication=major depressive disorder | ||
|adverseReactions=increased appetite, serum triglycerides raised, weight gain, constipation, xerostomia, ALT/SGPT level raised, asthenia, dizziness, somnolence, disturbance in thinking | |adverseReactions=increased appetite, serum triglycerides raised, weight gain, constipation, xerostomia, ALT/SGPT level raised, asthenia, dizziness, somnolence, disturbance in thinking | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;"> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">Suicidality and Antidepressant Drugs</span></b> | ||
|blackBoxWarningBody= | |blackBoxWarningBody=Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of mirtazapine orally disintegrating tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Mirtazapine orally disintegrating tablets are not approved for use in pediatric patients. | ||
|fdaLIADAdult=*Major depression | |fdaLIADAdult=*Major depression | ||
:*15 mg/day PO at bedtime, increase every 1-2 weeks to a max dose of 45 mg/day. | :*15 mg/day PO at bedtime, increase every 1-2 weeks to a max dose of 45 mg/day. | ||
| Line 20: | Line 20: | ||
|offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Mirtazapine in pediatric patients. | |offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Mirtazapine in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Mirtazapine in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Mirtazapine in pediatric patients. | ||
|useInPed=Safety and effectiveness of mirtazapine in the pediatric population have not been established | |||
|useInGeri=Following oral administration of mirtazapine tablets 20 mg/day for 7 days to subjects of varying ages (range, 25–74), oral clearance of mirtazapine was reduced in the elderly compared to the younger subjects. The differences were most striking in males, with a 40% lower clearance in elderly males compared to younger males, while the clearance in elderly females was only 10% lower compared to younger females. Caution is indicated in administering mirtazapine orally disintegrating tablets to elderly patients | |||
|useInGender=The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females vs. 26 hours for males) | |||
|useInRace=There have been no clinical studies to evaluate the effect of race on the pharmacokinetics of mirtazapine orally disintegrating tablets. | |||
|useInRenalImpair=The disposition of mirtazapine was studied in patients with varying degrees of renal function. Elimination of mirtazapine is correlated with creatinine clearance. Total body clearance of mirtazapine was reduced approximately 30% in patients with moderate (Clcr = 11–39 mL/min/1.73 m2) and approximately 50% in patients with severe (Clcr = <10 mL/min/1.73 m2) renal impairment when compared to normal subjects. Caution is indicated in administering mirtazapine orally disintegrating tablets to patients with compromised renal function. | |||
|useInHepaticImpair=Following a single 15-mg oral dose of mirtazapine, the oral clearance of mirtazapine was decreased by approximately 30% in hepatically impaired patients compared to subjects with normal hepatic function. Caution is indicated in administering mirtazapine orally disintegrating tablets to patients with compromised hepatic function. | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 87: | Line 93: | ||
| solubility = Soluble in [[methanol]] and [[chloroform]] | | solubility = Soluble in [[methanol]] and [[chloroform]] | ||
}} | }} | ||
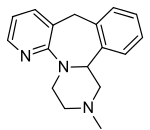
|structure=Mirtazapine orally disintegrating tablets USP are an orally administered drug. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated 1,2,3,4,10,14b-hexahydro-2-methylpyrazino [2,1-a] pyrido [2,3-c] benzazepine and has the empirical formula of C17H19N3. Its molecular weight is 265.36. The structural formula is the following and it is the racemic mixture: | |||
[[File:Mirt1.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Mirtazapine is a white to creamy white crystalline powder which is slightly soluble in water. Mirtazapine orally disintegrating tablets are available for oral administration as an orally disintegrating tablet containing 15 or 30 mg of mirtazapine. It disintegrates in the mouth within seconds after placement on the tongue, allowing its contents to be subsequently swallowed with or without water. Mirtazapine orally disintegrating tablets also contain the following inactive ingredients: aspartame powder, colloidal silicon dioxide, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, natural and artificial orange flavor, sodium stearyl fumarate and xylitol. | |||
|PD=* The mechanism of action of mirtazapine orally disintegrating tablets, as with other drugs effective in the treatment of major depressive disorder, is unknown. | |||
* Evidence gathered in preclinical studies suggests that mirtazapine enhances central noradrenergic and serotonergic activity. These studies have shown that mirtazapine acts as an antagonist at central presynaptic α2-adrenergic inhibitory autoreceptors and heteroreceptors, an action that is postulated to result in an increase in central noradrenergic and serotonergic activity. | |||
* Mirtazapine is a potent antagonist of 5-HT2 and 5-HT3 receptors. Mirtazapine has no significant affinity for the 5-HT1A and 5-HT1B receptors. | |||
* Mirtazapine is a potent antagonist of histamine (H1) receptors, a property that may explain its prominent sedative effects. | |||
* Mirtazapine is a moderate peripheral α1-adrenergic antagonist, a property that may explain the occasional orthostatic hypotension reported in association with its use. | |||
* Mirtazapine is a moderate antagonist at muscarinic receptors, a property that may explain the relatively low incidence of anticholinergic side effects associated with its use. | |||
|PK=* Mirtazapine orally disintegrating tablets are rapidly and completely absorbed following oral administration and have a half-life of about 20 to 40 hours. Peak plasma concentrations are reached within about 2 hours following an oral dose. The presence of food in the stomach has a minimal effect on both the rate and extent of absorption and does not require a dosage adjustment. Mirtazapine orally disintegrating tablets are bioequivalent to mirtazapine tablets. | |||
* Mirtazapine is extensively metabolized after oral administration. Major pathways of biotransformation are demethylation and hydroxylation followed by glucuronide conjugation. In vitro data from human liver microsomes indicate that cytochrome 2D6 and 1A2 are involved in the formation of the 8-hydroxy metabolite of mirtazapine, whereas cytochrome 3A is considered to be responsible for the formation of the N-desmethyl and N-oxide metabolite. Mirtazapine has an absolute bioavailability of about 50%. It is eliminated predominantly via urine (75%) with 15% in feces. Several unconjugated metabolites possess pharmacological activity but are present in the plasma at very low levels. The (–) enantiomer has an elimination half-life that is approximately twice as long as the (+) enantiomer and therefore achieves plasma levels that are about 3 times as high as that of the (+) enantiomer. | |||
* Plasma levels are linearly related to dose over a dose range of 15 to 80 mg. The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females vs. 26 hours for males). Steady state plasma levels of mirtazapine are attained within 5 days, with about 50% accumulation (accumulation ratio = 1.5). | |||
* Mirtazapine is approximately 85% bound to plasma proteins over a concentration range of 0.01 to 10 mcg/mL. | |||
|clinicalStudies=The efficacy of mirtazapine tablets as a treatment for major depressive disorder was established in 4 placebo-controlled, 6-week trials in adult outpatients meeting DSM-III criteria for major depressive disorder. Patients were titrated with mirtazapine from a dose range of 5 mg up to 35 mg/day. Overall, these studies demonstrated mirtazapine to be superior to placebo on at least 3 of the following 4 measures: 21-Item Hamilton Depression Rating Scale (HDRS) total score; HDRS Depressed Mood Item; CGI Severity score; and Montgomery and Asberg Depression Rating Scale (MADRS). Superiority of mirtazapine over placebo was also found for certain factors of the HDRS, including anxiety/somatization factor and sleep disturbance factor. The mean mirtazapine dose for patients who completed these 4 studies ranged from 21 to 32 mg/day. A fifth study of similar design utilized a higher dose (up to 50 mg) per day and also showed effectiveness. | |||
Examination of age and gender subsets of the population did not reveal any differential responsiveness on the basis of these subgroupings. | |||
In a longer-term study, patients meeting (DSM-IV) criteria for major depressive disorder who had responded during an initial 8 to 12 weeks of acute treatment on mirtazapine were randomized to continuation of mirtazapine or placebo for up to 40 weeks of observation for relapse. Response during the open phase was defined as having achieved a HAM-D 17 total score of ≤8 and a CGI-Improvement score of 1 or 2 at 2 consecutive visits beginning with week 6 of the 8 to 12 weeks in the open-label phase of the study. Relapse during the double-blind phase was determined by the individual investigators. Patients receiving continued mirtazapine treatment experienced significantly lower relapse rates over the subsequent 40 weeks compared to those receiving placebo. This pattern was demonstrated in both male and female patients. | |||
|alcohol=Alcohol-Mirtazapine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Mirtazapine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 00:06, 30 May 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mirtazapine is a noradrenergic and specific serotonergic antidepressant that is FDA approved for the {{{indicationType}}} of major depressive disorder. Common adverse reactions include increased appetite, serum triglycerides raised, weight gain, constipation, xerostomia, ALT/SGPT level raised, asthenia, dizziness, somnolence, disturbance in thinking.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Major depression
- 15 mg/day PO at bedtime, increase every 1-2 weeks to a max dose of 45 mg/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Mirtazapine in adult patients.
Non–Guideline-Supported Use
- Anxiety
- Cancer
- Dysthymia
- Obsessive-compulsive disorder
- Panic disorder
There is limited information about Off-Label Non–Guideline-Supported Use of Mirtazapine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety in pediatric patient has not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Mirtazapine in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Mirtazapine in pediatric patients.
Contraindications
There is limited information regarding Mirtazapine Contraindications in the drug label.
Warnings
There is limited information regarding Mirtazapine Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Mirtazapine Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Mirtazapine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Mirtazapine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Mirtazapine in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mirtazapine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mirtazapine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Mirtazapine in women who are nursing.
Pediatric Use
Safety and effectiveness of mirtazapine in the pediatric population have not been established
Geriatic Use
Following oral administration of mirtazapine tablets 20 mg/day for 7 days to subjects of varying ages (range, 25–74), oral clearance of mirtazapine was reduced in the elderly compared to the younger subjects. The differences were most striking in males, with a 40% lower clearance in elderly males compared to younger males, while the clearance in elderly females was only 10% lower compared to younger females. Caution is indicated in administering mirtazapine orally disintegrating tablets to elderly patients
Gender
The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females vs. 26 hours for males)
Race
There have been no clinical studies to evaluate the effect of race on the pharmacokinetics of mirtazapine orally disintegrating tablets.
Renal Impairment
The disposition of mirtazapine was studied in patients with varying degrees of renal function. Elimination of mirtazapine is correlated with creatinine clearance. Total body clearance of mirtazapine was reduced approximately 30% in patients with moderate (Clcr = 11–39 mL/min/1.73 m2) and approximately 50% in patients with severe (Clcr = <10 mL/min/1.73 m2) renal impairment when compared to normal subjects. Caution is indicated in administering mirtazapine orally disintegrating tablets to patients with compromised renal function.
Hepatic Impairment
Following a single 15-mg oral dose of mirtazapine, the oral clearance of mirtazapine was decreased by approximately 30% in hepatically impaired patients compared to subjects with normal hepatic function. Caution is indicated in administering mirtazapine orally disintegrating tablets to patients with compromised hepatic function.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mirtazapine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mirtazapine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Mirtazapine Administration in the drug label.
Monitoring
There is limited information regarding Mirtazapine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Mirtazapine and IV administrations.
Overdosage
There is limited information regarding Mirtazapine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Mirtazapine Mechanism of Action in the drug label.
Structure
Mirtazapine orally disintegrating tablets USP are an orally administered drug. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated 1,2,3,4,10,14b-hexahydro-2-methylpyrazino [2,1-a] pyrido [2,3-c] benzazepine and has the empirical formula of C17H19N3. Its molecular weight is 265.36. The structural formula is the following and it is the racemic mixture:

Mirtazapine is a white to creamy white crystalline powder which is slightly soluble in water. Mirtazapine orally disintegrating tablets are available for oral administration as an orally disintegrating tablet containing 15 or 30 mg of mirtazapine. It disintegrates in the mouth within seconds after placement on the tongue, allowing its contents to be subsequently swallowed with or without water. Mirtazapine orally disintegrating tablets also contain the following inactive ingredients: aspartame powder, colloidal silicon dioxide, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, natural and artificial orange flavor, sodium stearyl fumarate and xylitol.
Pharmacodynamics
- The mechanism of action of mirtazapine orally disintegrating tablets, as with other drugs effective in the treatment of major depressive disorder, is unknown.
- Evidence gathered in preclinical studies suggests that mirtazapine enhances central noradrenergic and serotonergic activity. These studies have shown that mirtazapine acts as an antagonist at central presynaptic α2-adrenergic inhibitory autoreceptors and heteroreceptors, an action that is postulated to result in an increase in central noradrenergic and serotonergic activity.
- Mirtazapine is a potent antagonist of 5-HT2 and 5-HT3 receptors. Mirtazapine has no significant affinity for the 5-HT1A and 5-HT1B receptors.
- Mirtazapine is a potent antagonist of histamine (H1) receptors, a property that may explain its prominent sedative effects.
- Mirtazapine is a moderate peripheral α1-adrenergic antagonist, a property that may explain the occasional orthostatic hypotension reported in association with its use.
- Mirtazapine is a moderate antagonist at muscarinic receptors, a property that may explain the relatively low incidence of anticholinergic side effects associated with its use.
Pharmacokinetics
- Mirtazapine orally disintegrating tablets are rapidly and completely absorbed following oral administration and have a half-life of about 20 to 40 hours. Peak plasma concentrations are reached within about 2 hours following an oral dose. The presence of food in the stomach has a minimal effect on both the rate and extent of absorption and does not require a dosage adjustment. Mirtazapine orally disintegrating tablets are bioequivalent to mirtazapine tablets.
- Mirtazapine is extensively metabolized after oral administration. Major pathways of biotransformation are demethylation and hydroxylation followed by glucuronide conjugation. In vitro data from human liver microsomes indicate that cytochrome 2D6 and 1A2 are involved in the formation of the 8-hydroxy metabolite of mirtazapine, whereas cytochrome 3A is considered to be responsible for the formation of the N-desmethyl and N-oxide metabolite. Mirtazapine has an absolute bioavailability of about 50%. It is eliminated predominantly via urine (75%) with 15% in feces. Several unconjugated metabolites possess pharmacological activity but are present in the plasma at very low levels. The (–) enantiomer has an elimination half-life that is approximately twice as long as the (+) enantiomer and therefore achieves plasma levels that are about 3 times as high as that of the (+) enantiomer.
- Plasma levels are linearly related to dose over a dose range of 15 to 80 mg. The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females vs. 26 hours for males). Steady state plasma levels of mirtazapine are attained within 5 days, with about 50% accumulation (accumulation ratio = 1.5).
- Mirtazapine is approximately 85% bound to plasma proteins over a concentration range of 0.01 to 10 mcg/mL.
Nonclinical Toxicology
There is limited information regarding Mirtazapine Nonclinical Toxicology in the drug label.
Clinical Studies
The efficacy of mirtazapine tablets as a treatment for major depressive disorder was established in 4 placebo-controlled, 6-week trials in adult outpatients meeting DSM-III criteria for major depressive disorder. Patients were titrated with mirtazapine from a dose range of 5 mg up to 35 mg/day. Overall, these studies demonstrated mirtazapine to be superior to placebo on at least 3 of the following 4 measures: 21-Item Hamilton Depression Rating Scale (HDRS) total score; HDRS Depressed Mood Item; CGI Severity score; and Montgomery and Asberg Depression Rating Scale (MADRS). Superiority of mirtazapine over placebo was also found for certain factors of the HDRS, including anxiety/somatization factor and sleep disturbance factor. The mean mirtazapine dose for patients who completed these 4 studies ranged from 21 to 32 mg/day. A fifth study of similar design utilized a higher dose (up to 50 mg) per day and also showed effectiveness.
Examination of age and gender subsets of the population did not reveal any differential responsiveness on the basis of these subgroupings.
In a longer-term study, patients meeting (DSM-IV) criteria for major depressive disorder who had responded during an initial 8 to 12 weeks of acute treatment on mirtazapine were randomized to continuation of mirtazapine or placebo for up to 40 weeks of observation for relapse. Response during the open phase was defined as having achieved a HAM-D 17 total score of ≤8 and a CGI-Improvement score of 1 or 2 at 2 consecutive visits beginning with week 6 of the 8 to 12 weeks in the open-label phase of the study. Relapse during the double-blind phase was determined by the individual investigators. Patients receiving continued mirtazapine treatment experienced significantly lower relapse rates over the subsequent 40 weeks compared to those receiving placebo. This pattern was demonstrated in both male and female patients.
How Supplied
There is limited information regarding Mirtazapine How Supplied in the drug label.
Storage
There is limited information regarding Mirtazapine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mirtazapine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mirtazapine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Mirtazapine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Mirtazapine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Mirtazapine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Mirtazapine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Timmer, CJ; Sitsen, JM; Delbressine, LP (June 2000). "Clinical pharmacokinetics of mirtazapine". Clinical Pharmacokinetics. 38 (6): 461–74. doi:10.2165/00003088-200038060-00001. PMID 10885584.