Methaqualone
|
WikiDoc Resources for Methaqualone |
|
Articles |
|---|
|
Most recent articles on Methaqualone Most cited articles on Methaqualone |
|
Media |
|
Powerpoint slides on Methaqualone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Methaqualone at Clinical Trials.gov Clinical Trials on Methaqualone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Methaqualone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Methaqualone Discussion groups on Methaqualone Patient Handouts on Methaqualone Directions to Hospitals Treating Methaqualone Risk calculators and risk factors for Methaqualone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Methaqualone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
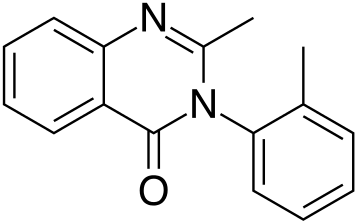 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H14N2O |
| Molar mass | 250.30 g/mol |
| 3D model (JSmol) | |
| Melting point | 113 °C (235.4 °F) |
| |
| |
| (verify) | |
Methaqualone, brand name Quaalude (sometimes stylized Quāālude[1]) /ˈkweɪluːd/ in the US and Mandrax in the UK, is a central nervous system (CNS) depressant of the quinazolinone class that acts as a sedative and hypnotic.
The sedative–hypnotic activity of methaqualone was first noted by Indian researchers in the 1950s and in 1962 methaqualone itself was patented in the US by Wallace and Tiernan.[2] Its use peaked in the early 1970s as a hypnotic, for the treatment of insomnia, and as a sedative and muscle relaxant. It is still produced and used clandestinely as a recreational drug throughout the world. The drug was popular in the 1970s disco club scene.
History
Methaqualone was first synthesized in India in 1951 by Indra Kishore Kacker and Syed Hussain Zaheer, for use as an antimalarial drug.[3][4] By 1965 it was the most commonly prescribed sedative in Britain, where it has been sold legally under the names Malsed, Malsedin, and Renoval. In 1965 a methaqualone/antihistamine combination was sold as the sedative drug Mandrax, by Roussel Laboratories (now part of Sanofi-Aventis). In 1972 it was the sixth-bestselling sedative in the US,[5] where it was legal under the brand name Quaalude.
Quaalude in the United States was originally manufactured in 1965 by the Fort Washington, Pennsylvania, based pharmaceutical firm William H. Rorer Inc. The drug name "Quaalude" shared a stylistic reference to another drug marketed by the firm, Maalox, and was a portmanteau of the phrase "quiet interlude".[6]
In 1978, Rorer sold the rights to manufacture Quaalude to the Lemmon Company of Sellersville, Pennsylvania. At that time, Rorer chairman John Eckman commented on Quaalude's bad reputation stemming from illegal manufacture and use of methaqualone, and illegal sale and use of legally prescribed Quaalude:
Both companies still regarded Quaalude as an excellent sleeping drug. Lemmon, well aware of Quaalude's public image problems, used advertisements in medical journals to urge physicians "not to permit the abuses of illegal users to deprive a legitimate patient of the drug". Lemmon also marketed a small quantity under another name, Mequin, so doctors could prescribe the drug without the negative connotations.
The rights to Quaalude were held by the JB Roerig & Company division of Pfizer, before the drug was discontinued in the United States in 1985, mainly due to its psychological addictiveness and recreational use.[7]
Uses
Medical
Methaqualone is a depressant that increases the activity of the GABA receptors in the brain and nervous system. When GABA activity is increased, blood pressure drops and the breathing and pulse rates slow, leading to a state of deep relaxation. These properties explain why methaqualone was originally mainly prescribed for insomnia.[8]
Methaqualone peaks in the bloodstream within several hours, with a half-life of 20–60 hours. Regular users build up a physical tolerance, requiring larger doses for the same effect. Overdose can lead to nervous system shutdown, coma and death.[9]
Methaqualone is not recommended for use while pregnant and is in pregnancy category D.[10] In Canada, methaqualone is listed in Schedule III of the Controlled Drugs and Substances Act and requires a prescription.[11][12] It is banned in India.[13]
Recreational
Methaqualone became increasingly popular as a recreational drug in the late 1960s and early 1970s, known variously as 'ludes or sopers (also soapers) in the U.S. and mandrakes and mandies in the UK and Australia.
The drug was often used by people who went dancing at glam rock clubs in the early 1970s and at discos in the late 1970s. (One slang term for Quaalude was disco biscuits.) In the mid-1970s there were bars in Manhattan called juice bars that only served non-alcoholic drinks that catered to people who liked to dance on methaqualone.[14]
Smoking methaqualone, either by itself or as an adulterant added to various legal and illegal smoking mixtures, gained popularity in the US for a few years during the mid-1970s. Because the various binders and inert ingredients that were contained in the pill form were toxic when smoked, this practice was roundly decried by the medical community as a serious health risk. Smoking methaqualone pills can lead to emphysema and other chronic lung disorders, most notably talcosis.
The drug was more tightly regulated in Britain under the Misuse of Drugs Act 1971 and in the U.S. from 1973. It was withdrawn from many developed markets in the early 1980s. In the United States it was withdrawn in 1982 and made a Schedule I drug in 1984. It has a DEA ACSCN of 2565 and in 2013 the aggregate annual manufacturing quota for the US was 10 grams. Mention of its possible use in some types of cancer and AIDS has periodically appeared in the literature since the late 1980s; research does not appear to have reached an advanced stage. The DEA has also added the methaqualone analogue mecloqualone (also a result of some incomplete clandestine syntheses) to Schedule I as ACSCN 2572, and zero manufacturing quota.
Gene Haislip, the former head of the Chemical Control Division of the Drug Enforcement Administration (DEA), told the PBS documentary program Frontline: "We beat 'em." By working with governments and manufacturers around the world, the DEA was able to halt production and, Haislip says, "eliminated the problem".[15][16][17]
Methaqualone was manufactured in the United States under the name Quaalude by the pharmaceutical firms Rorer and Lemmon with the numbers 714 stamped on the tablet, so people often referred to Quaalude as 714s, "Lemmons", or "Lemmon 7s". Methaqualone was also manufactured in the US under the trade names Sopor and Parest. After the legal manufacture of the drug ended in the United States in 1982, underground laboratories in Mexico continued illegal manufacture of methaqualone all through the 1980s, continuing the use of the "714" stamp, until their popularity waned in the early 1990s.
Methaqualone is one of the most commonly used recreational drugs in South Africa.[18][19] It is also popular elsewhere in Africa and in India.[19] Commonly known as Mandrax, M-pills, buttons, or smarties, a mixture of crushed mandrax and cannabis is smoked, usually through a smoking pipe made from the neck of a broken bottle. Wouter Basson (q.v.) produced 200 kilos of powder methaqualone and 100 000 Mandrax tablets as part of the massive drug cache that disappeared into the underground after the end of Apartheid and replacement of the National Party by the ANC in office, along with the famous "Basson Brownies", capsules of pure MDMA. The drugs were manufactured under the aegis of Project Coast, which included research into the use of anticholinergics, cocaine, empathogens, and depressants as riot-control agents.
Effects
Effects can include drowsiness, reduced heart rate, reduced respiration, increased sexual arousal (aphrodisia), and paresthesias (numbness of the fingers and toes). Larger doses can bring about respiratory depression, slurred speech, headache, and photophobia (a symptom of excessive sensitivity to light).
Overdose
An overdose can cause delirium, convulsions, hypertonia, hyperreflexia, vomiting, kidney failure, coma, and death through cardiac or respiratory arrest. It resembles barbiturate poisoning, but with increased motor difficulties and a lower incidence of cardiac or respiratory depression. The standard one tablet adult dosage of Quaalude was 75 mg; a dose of 8000 mg is lethal. However, a dose as little as 2000 mg could also be lethal, especially if taken with an alcoholic beverage.
Drug testing
Urine drug test of gas-liquid chromatography (GLC) confirmation up to 72 hours after the last intake is a practical way of detecting methaqualone use.[20]
To avoid a false positive Substance Abuse and Mental Health Services Administration (SAMHSA) determined the initial cut off level of 300 ng/ml for forensic and workplace drug testing of methaqualone. Only after testing at over 200 ng/ml is a person be considered positive, and possibly subject to penalties or workplace disciplinary action.[21] As reported by Quest Diagnostics in 2011, methaqualone had a 0% positive drug testing rate in the US, making it one of the least used recreational drugs in that year.[22]
See also
- Cloroqualone
- Diproqualone
- Etaqualone
- Mebroqualone
- Mecloqualone
- Methylmethaqualone
- Gamma-Aminobutyric acid
References
- ↑ Rile, Karen (1983). Winter Music (First ed.). Boston and Toronto: Little, Brown and Company. pp. 41, 59. ISBN 0-316-74657-6.
- ↑ US Patent 3135659 – Hydroxy and Alkoxy Aryl Quinazolones
- ↑ van Zyl, Etienne F. (2001). "A survey of reported synthesis of methaqualone and some positional and structural isomers". Forensic Science International. 122 (2–3): 142–149. doi:10.1016/S0379-0738(01)00484-4.
- ↑ Potential Analgesics. Part I. Synthesis of substituted 4-quinazolones, I. K. Kacker and S. H. Zaheer, J. Ind. Chem. Soc. 28 (1951), pp. 344–346.
- ↑ GC/MS Assays for Abused Drugs in Body fluids, p. 39
- ↑ "Dividends: Dropping the Last 'Lude". Time. 28 November 1983. Retrieved 16 August 2013.
- ↑ Silverstein, Shel. "Quaaludes Again". Captain Wayne's Mad Music.com.
- ↑ "methaqualone reference". Enotes.
- ↑ "recreational drugs tranquilizers". drug library eu.
- ↑ Drug Safety. "Methaqualone in Pregnancy and Breastfeeding". Retrieved 15 August 2012.
- ↑ "Search National Drug Schedule".
- ↑ "Schedule III, Controlled Drugs and Substances Act". Her Majesty in Right of Canada. Retrieved 29 November 2014.
- ↑ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Retrieved 2013-09-17.
- ↑ Lawrence Young (31 January 2010). "METHAQUALONE". Drug Text. International Substance Use Library. Retrieved 27 July 2012.
- ↑ "The Meth Epidemic – Haislip discusses parallels to current Methamphetamine epidemic".
- ↑ Ferns, Sean, "Lecture: Gene Haislip : The Chemical Connection: A Historical Perspective on Chemical Control", Drug Enforcement Administration Museum Lecture Series, Arlington, Virginia, October 25, 2007
- ↑ Piccini, Sara, "DRUG WARRIOR: THE DEA’S GENE HAISLIP ’60, B.C.L. ’63 BATTLED WORLDWIDE AGAINST THE ILLEGAL DRUG TRADE – AND SCORED A RARE VICTORY", William & Mary Alumni Magazine, The College of William & Mary, Spring 2010
- ↑ "Mandrax". DrugAware. Reality Media. 2003. Retrieved 2009-08-13.
- ↑ 19.0 19.1 "Treatment for methaqualone dependence in adults". Reviews (2): CD004146. 2005. doi:10.1002/14651858.CD004146.pub2. PMID 15846700.
- ↑ "methaqualone drug testing". National Institute of Health.
- ↑ "methaqualone drug testing". Testcountry.
- ↑ "workplace drug test positive rates". quest diagnostics.