Methandrostenolone: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
Rabin Bista (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{drugbox | | {{drugbox | | ||
| IUPAC_name = (8''S'',9''S'',10''S'',13''S'',14''S'',17''S'')-17-hydroxy-10,<br>13,17-trimethyl-7,8,9,11,12,14,15,16-<br>octahydro-6''H''-cyclopenta[a]phenanthren-3-one | | IUPAC_name = (8''S'',9''S'',10''S'',13''S'',14''S'',17''S'')-17-hydroxy-10,<br>13,17-trimethyl-7,8,9,11,12,14,15,16-<br>octahydro-6''H''-cyclopenta[a]phenanthren-3-one | ||
| Line 20: | Line 18: | ||
| routes_of_administration = Oral | | routes_of_administration = Oral | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | {{SI}} | ||
'''Methandrostenolone''' ('''Dianabol''') is an [[anabolic steroid]] originally developed | {{CMG}} | ||
== Overview == | |||
'''Methandrostenolone''' (trade names '''Averbol''', '''Dianabol''', '''Danabol'''), also known as '''metandienone''' ([[International Nonproprietary Name|INN]]), '''methandienone''', or informally as '''dianabol''', is an orally-effective [[anabolic steroid]] originally developed in [[Germany]] and released in the [[United States|US]] in the early 1960s by [[Ciba Specialty Chemicals]].<ref>{{cite journal |author=Yesalis CE, Anderson WA, Buckley WE, Wright JE |title=Incidence of the nonmedical use of anabolic-androgenic steroids |journal=NIDA Res. Monogr. |volume=102 |pages=97–112 |year=1990 |pmid=2079979 |url=http://www.drugabuse.gov/pdf/monographs/102.pdf}}</ref><ref>{{cite journal |author=Fair JD |title=Isometrics or Steroids? Exploring New Frontiers Of Strength in the Early 1960s |journal=Journal of Sport History |volume=20 |issue=1 |pages=1–24 |year=1993 |url=http://www.la84foundation.org/SportsLibrary/JSH/JSH1993/JSH2001/jsh2001b.pdf}}</ref> Methandrostenolone is a controlled substance in the United States<ref>{{cite journal |author=Drug Enforcement Administration |title=Controlled Substances, Alphabetical Order |url=http://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf}}</ref> and Western Europe and remains popular among bodybuilders. An injectable form is sold online from United States based companies. Methandrostenolone is readily available without a prescription in countries such as [[Mexico]] (under the trade name Reforvit-b), and is also being manufactured in [[Asia]] and many [[East Europe]]an countries. | |||
==Biophysiology== | |||
Methandrostenolone binds tightly to the [[androgen receptor]] in order to exert its effects.<ref>{{cite journal |author=Roselli CE |title=The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area |journal=Brain Res. |volume=792 |issue=2 |pages=271–6 |date=May 1998 |pmid=9593936 |url=http://linkinghub.elsevier.com/retrieve/pii/S0006-8993(98)00148-6 |doi=10.1016/S0006-8993(98)00148-6}}</ref> These include dramatic increases in [[protein biosynthesis|protein synthesis]], [[glycogenolysis]], and muscle strength over a short space of time.{{medcn|date=April 2014}} Side effects such as [[gynecomastia]], high [[blood pressure]], [[Acne vulgaris|acne]] and [[male pattern baldness]] may begin to occur. The drug causes severe masculinising effects in women even at low doses. In addition, it is metabolized into methylestradiol by [[aromatase]]. This means that without the administration of [[aromatase]] inhibitors such as [[anastrozole]] or [[aminoglutethimide]], [[estrogen]]ic effects will appear over time in men. Many users will combat the [[estrogen]]ic side effects with [[Arimidex]], [[Nolvadex]] or [[Clomid]]. In addition, as with other 17α-alkylated steroids, the use of methandrostenolone over extended periods of time can result in [[cirrhosis|liver damage]] without appropriate care. | |||
The 17α-methylation of the steroid does allow it to pass through the liver with only a small portion of it broken down (hence causing the aforementioned damage to the liver) allowing it to be effective when taken orally. It also has the effect of decreasing the steroid's affinity for [[sex hormone binding globulin]], a protein that de-activates steroid molecules and prevents them from further reactions with the body. As a result, methandrostenolone is significantly more active than an equivalent quantity of [[testosterone]], resulting in rapid growth of muscle tissue. However, the concomitant elevation in [[estrogen]] levels - a result of the [[aromatization]] of methandrostenolone - results in significant water retention. This gives the appearance of bad gains in mass and strength, which prove to be temporary once the steroid is discontinued and water weight drops. Because of this, it is often used by bodybuilders only at the start of a "steroid cycle", to facilitate rapid strength increases and the appearance of great size, while compounds such as testosterone cypionate or testosterone enanthate with long acting [[esters]] build up in the body to an appreciable amount capable of supporting [[anabolic]] function on their own. | |||
==Usage== | |||
In the early 1960s, doctors commonly prescribed | ===As a tonic=== | ||
[[File:Metandinone ph 1.png|right|250px]] | |||
[[File:Metandinone ph 2.png|right|250px]] | |||
In the early 1960s, doctors commonly prescribed 3 tablets per day for women as a [[Pharmaceutical drug#For nutrition|tonic]]. This use was quickly discontinued upon discovery of the heavily masculinising effects of methandrostenolone. | |||
===Bodybuilding=== | |||
Despite the lack of any known therapeutic applications, the drug remained legal until 2001.{{citation needed|date=April 2013}} The United States Congress added certain kinds of steroids which may or may not include methandrostenolone {{Clarify|date=April 2013}} to the Controlled Substances Act as an amendment known as the Anabolic Steroid Control Act of 1990.{{citation needed|date=April 2013}} This act placed steroids in the same category as some amphetamines as a "Schedule III" drug and possession of these drugs results in a felony. It is used by bodybuilders and methandrostenolone continues to be used illegally to this day, typically being combined (stacked) with injectable compounds, such as [[testosterone propionate]], [[testosterone enanthate|enanthate]], [[testosterone cypionate|cypionate]] as well as other injectable drugs like [[trenbolone acetate]].{{cn|date=April 2014}} | |||
== | Several successful athletes and professional bodybuilders have come forward and admitted long-term methandrostenolone use before the drug was banned, including [[Arnold Schwarzenegger]].<ref name="ST">Steve Theunissen: [http://hjem.get2net.dk/JamesBond/www/artikler/steroidemisbrug/arnoldandsteroids.htm Arnold & Steroids: Truth Revealed] 2002</ref>{{dead link|date=August 2014}}<ref name="SOI">{{cite web|url=http://www.fitnessprat.no/archive/index.php/t-332.html |title=Interview with Sergio Oliva |publisher=Fitnessprat.no |date=2010-10-18 |accessdate=2012-02-13}}</ref>{{dead link|date=August 2014}} Other steroids stacked with methandrostenolone are primarily, if not always, injectable compounds such as [[testosterone]], [[trenbolone]] and [[nandrolone]].{{cn|date=April 2014}} Large doses and long-term use of methandrostenolone have been associated with eccentric [[left ventricular hypertrophy]] which presents substantially increased risks of [[cardiomyopathy]] if and when the hypertrophy [[atrophies]].{{medcn|date=April 2014}} Athleticism is typically associated with left-ventricular hypertrophy however natural athleticism generally presents concentric left ventricular growth which is not linked to an increased risk of cardiomyopathy.{{medcn|date=April 2014}} | ||
=== Detection of use === | |||
Methandrostenolone is subject to extensive hepatic biotransformation by a variety of enzymatic pathways. The primary urinary metabolites are detectable for up to 3 days, and a recently discovered hydroxymethyl metabolite is found in urine for up to 19 days after a single 5 mg oral dose.<ref>Schänzer W, Geyer H, Fusshöller G, Halatcheva N, Kohler M, Parr MK, Guddat S, Thomas A, Thevis M. Mass spectrometric identification and characterization of a new long-term metabolite of metandienone in human urine. Rapid Commun. Mass Spectrom. 20: 2252-8, 2008.</ref> Several of the metabolites are unique to methandrostenolone. Methods for detection in urine specimens usually involve gas chromatography-mass spectrometry.<ref>R. Baselt, ''Disposition of Toxic Drugs and Chemicals in Man'', 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 952-954.</ref><ref>Fragkaki AG, Angelis YS, Tsantili-Kakoulidou A, Koupparis M, Georgakopoulos C. Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. J. Steroid Biochem. Mol. Biol. 115: 44-61, 2009.</ref> | |||
==History== | |||
{{See also| Weightlifting at the 1960 Summer Olympics}} | |||
For a period of time [[John Bosley Ziegler]] worked at the [[Novartis|Ciba Pharmaceutical]] company, who supplied testosterone for experimental purposes. In the early 1950s his patients included people suffering from burns, as well as the seriously injured or handicapped. In 1954 he administered testosterone, for a period of less than 6 weeks, to several high-level competitive bodybuilders on an experimental basis, but had disappointing results. Dissatisfied and possibly overburdened with patients, he distanced himself from research into [[performance-enhancing drugs]] until May 1960, or possibly as early as 1959 (conflicting testimonials).{{Citation needed|date=January 2010}} | |||
By the time of the 1960 European Championships in [[Milan]] he was understandably suspicious of the [[Russia]]ns - "the Russians are giving their athletes something." Therefore, he asked [[John Grimek]] to propose to his chief, [[Bob Hoffman (promoter)|Bob Hoffman]] that steroids be administered to members of the [[United States|American]] [[Olympic Games|Olympic]] team. Mr. Hoffman, however, was cautious and later remarked it was "too close to give to the men who will represent the [[USA]]". According to Grimek, "Apparently, he doesn’t think it will do that much good, and may even have detrimental effects , . . .He appears doubtful." Instead, Dianabol was given to two lower level lifters to investigate its effectiveness and safety. After that, Hoffmann retracted his decision and Dianabol was administered to certain Weightlifters on the team.{{Citation needed|date=August 2010}}<ref>{{cite web|url=http://startingstrength.com/articles/ultimate_exercise_starr.pdf |title=The Ultimate Strength Exercise 1, Bill Starr |format=PDF |date= |accessdate=2012-02-13}}</ref><ref>{{cite web|url=http://startingstrength.com/articles/ultimate_exercise_2_starr.pdf |title=The Ultimate Strength Exercise 2, Bill Starr |format=PDF |date= |accessdate=2012-02-13}}</ref> | |||
==Synthesis== | |||
[[File:Metandinone synthesis.png|center|700px]] | |||
Treatment of [[methyltestosterone]] with [[selenium dioxide]], removes hydrogen from ring A to form a new double bond at C1, yielding methandrostenolone. | |||
==Footnotes== | |||
{{Reflist|2}} | |||
==Other references== | |||
*{{cite journal |author=Wilder EM |title=Death due to Liver Failure Following the Use of Methandrostenolone |journal=Can Med Assoc J |volume=87 |issue= 14|pages=768–9 |date=October 1962 |pmid=14000685 |pmc=1849648 }} | |||
*{{cite journal |author=Foss GL |title=Some Experiences with a New Anabolic Steroid (Methandrostenolone) |journal=Br Med J |volume=1 |issue=5182 |pages=1300–5 |date=April 1960 |pmid=13824087 |pmc=1967563 |doi=10.1136/bmj.1.5182.1300 }} | |||
*{{cite journal |author=Zingg W |title=The Effect of Methandrostenolone on Nitrogen Excretion Following Open-Heart Surgery |journal=Can Med Assoc J |volume=93 |issue=15 |pages=816–7 |date=October 1965 |pmid=5318132 |pmc=1928923 }} | |||
{{Anabolic steroids}} | {{Anabolic steroids}} | ||
[[Category: | {{Androgenics}} | ||
[[Category:drug]] | |||
[[Category:Anabolic steroids]] | [[Category:Anabolic steroids]] | ||
Latest revision as of 17:54, 15 April 2015
| Error creating thumbnail: File missing | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 4.5-6 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.441 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Methandrostenolone (trade names Averbol, Dianabol, Danabol), also known as metandienone (INN), methandienone, or informally as dianabol, is an orally-effective anabolic steroid originally developed in Germany and released in the US in the early 1960s by Ciba Specialty Chemicals.[1][2] Methandrostenolone is a controlled substance in the United States[3] and Western Europe and remains popular among bodybuilders. An injectable form is sold online from United States based companies. Methandrostenolone is readily available without a prescription in countries such as Mexico (under the trade name Reforvit-b), and is also being manufactured in Asia and many East European countries.
Biophysiology
Methandrostenolone binds tightly to the androgen receptor in order to exert its effects.[4] These include dramatic increases in protein synthesis, glycogenolysis, and muscle strength over a short space of time.Template:Medcn Side effects such as gynecomastia, high blood pressure, acne and male pattern baldness may begin to occur. The drug causes severe masculinising effects in women even at low doses. In addition, it is metabolized into methylestradiol by aromatase. This means that without the administration of aromatase inhibitors such as anastrozole or aminoglutethimide, estrogenic effects will appear over time in men. Many users will combat the estrogenic side effects with Arimidex, Nolvadex or Clomid. In addition, as with other 17α-alkylated steroids, the use of methandrostenolone over extended periods of time can result in liver damage without appropriate care.
The 17α-methylation of the steroid does allow it to pass through the liver with only a small portion of it broken down (hence causing the aforementioned damage to the liver) allowing it to be effective when taken orally. It also has the effect of decreasing the steroid's affinity for sex hormone binding globulin, a protein that de-activates steroid molecules and prevents them from further reactions with the body. As a result, methandrostenolone is significantly more active than an equivalent quantity of testosterone, resulting in rapid growth of muscle tissue. However, the concomitant elevation in estrogen levels - a result of the aromatization of methandrostenolone - results in significant water retention. This gives the appearance of bad gains in mass and strength, which prove to be temporary once the steroid is discontinued and water weight drops. Because of this, it is often used by bodybuilders only at the start of a "steroid cycle", to facilitate rapid strength increases and the appearance of great size, while compounds such as testosterone cypionate or testosterone enanthate with long acting esters build up in the body to an appreciable amount capable of supporting anabolic function on their own.
Usage
As a tonic


In the early 1960s, doctors commonly prescribed 3 tablets per day for women as a tonic. This use was quickly discontinued upon discovery of the heavily masculinising effects of methandrostenolone.
Bodybuilding
Despite the lack of any known therapeutic applications, the drug remained legal until 2001.[citation needed] The United States Congress added certain kinds of steroids which may or may not include methandrostenolone[clarification needed] to the Controlled Substances Act as an amendment known as the Anabolic Steroid Control Act of 1990.[citation needed] This act placed steroids in the same category as some amphetamines as a "Schedule III" drug and possession of these drugs results in a felony. It is used by bodybuilders and methandrostenolone continues to be used illegally to this day, typically being combined (stacked) with injectable compounds, such as testosterone propionate, enanthate, cypionate as well as other injectable drugs like trenbolone acetate.[citation needed]
Several successful athletes and professional bodybuilders have come forward and admitted long-term methandrostenolone use before the drug was banned, including Arnold Schwarzenegger.[5][dead link][6][dead link] Other steroids stacked with methandrostenolone are primarily, if not always, injectable compounds such as testosterone, trenbolone and nandrolone.[citation needed] Large doses and long-term use of methandrostenolone have been associated with eccentric left ventricular hypertrophy which presents substantially increased risks of cardiomyopathy if and when the hypertrophy atrophies.Template:Medcn Athleticism is typically associated with left-ventricular hypertrophy however natural athleticism generally presents concentric left ventricular growth which is not linked to an increased risk of cardiomyopathy.Template:Medcn
Detection of use
Methandrostenolone is subject to extensive hepatic biotransformation by a variety of enzymatic pathways. The primary urinary metabolites are detectable for up to 3 days, and a recently discovered hydroxymethyl metabolite is found in urine for up to 19 days after a single 5 mg oral dose.[7] Several of the metabolites are unique to methandrostenolone. Methods for detection in urine specimens usually involve gas chromatography-mass spectrometry.[8][9]
History
For a period of time John Bosley Ziegler worked at the Ciba Pharmaceutical company, who supplied testosterone for experimental purposes. In the early 1950s his patients included people suffering from burns, as well as the seriously injured or handicapped. In 1954 he administered testosterone, for a period of less than 6 weeks, to several high-level competitive bodybuilders on an experimental basis, but had disappointing results. Dissatisfied and possibly overburdened with patients, he distanced himself from research into performance-enhancing drugs until May 1960, or possibly as early as 1959 (conflicting testimonials).[citation needed]
By the time of the 1960 European Championships in Milan he was understandably suspicious of the Russians - "the Russians are giving their athletes something." Therefore, he asked John Grimek to propose to his chief, Bob Hoffman that steroids be administered to members of the American Olympic team. Mr. Hoffman, however, was cautious and later remarked it was "too close to give to the men who will represent the USA". According to Grimek, "Apparently, he doesn’t think it will do that much good, and may even have detrimental effects , . . .He appears doubtful." Instead, Dianabol was given to two lower level lifters to investigate its effectiveness and safety. After that, Hoffmann retracted his decision and Dianabol was administered to certain Weightlifters on the team.[citation needed][10][11]
Synthesis
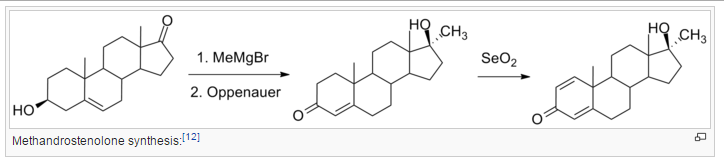
Treatment of methyltestosterone with selenium dioxide, removes hydrogen from ring A to form a new double bond at C1, yielding methandrostenolone.
Footnotes
- ↑ Yesalis CE, Anderson WA, Buckley WE, Wright JE (1990). "Incidence of the nonmedical use of anabolic-androgenic steroids" (PDF). NIDA Res. Monogr. 102: 97–112. PMID 2079979.
- ↑ Fair JD (1993). "Isometrics or Steroids? Exploring New Frontiers Of Strength in the Early 1960s" (PDF). Journal of Sport History. 20 (1): 1–24.
- ↑ Drug Enforcement Administration. "Controlled Substances, Alphabetical Order" (PDF).
- ↑ Roselli CE (May 1998). "The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area". Brain Res. 792 (2): 271–6. doi:10.1016/S0006-8993(98)00148-6. PMID 9593936.
- ↑ Steve Theunissen: Arnold & Steroids: Truth Revealed 2002
- ↑ "Interview with Sergio Oliva". Fitnessprat.no. 2010-10-18. Retrieved 2012-02-13.
- ↑ Schänzer W, Geyer H, Fusshöller G, Halatcheva N, Kohler M, Parr MK, Guddat S, Thomas A, Thevis M. Mass spectrometric identification and characterization of a new long-term metabolite of metandienone in human urine. Rapid Commun. Mass Spectrom. 20: 2252-8, 2008.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 952-954.
- ↑ Fragkaki AG, Angelis YS, Tsantili-Kakoulidou A, Koupparis M, Georgakopoulos C. Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. J. Steroid Biochem. Mol. Biol. 115: 44-61, 2009.
- ↑ "The Ultimate Strength Exercise 1, Bill Starr" (PDF). Retrieved 2012-02-13.
- ↑ "The Ultimate Strength Exercise 2, Bill Starr" (PDF). Retrieved 2012-02-13.
Other references
- Wilder EM (October 1962). "Death due to Liver Failure Following the Use of Methandrostenolone". Can Med Assoc J. 87 (14): 768–9. PMC 1849648. PMID 14000685.
- Foss GL (April 1960). "Some Experiences with a New Anabolic Steroid (Methandrostenolone)". Br Med J. 1 (5182): 1300–5. doi:10.1136/bmj.1.5182.1300. PMC 1967563. PMID 13824087.
- Zingg W (October 1965). "The Effect of Methandrostenolone on Nitrogen Excretion Following Open-Heart Surgery". Can Med Assoc J. 93 (15): 816–7. PMC 1928923. PMID 5318132.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from April 2013
- Articles with invalid date parameter in template
- Wikipedia articles needing clarification from April 2013
- Articles with unsourced statements from April 2014
- All articles with dead external links
- Articles with dead external links from August 2014
- Articles with unsourced statements from January 2010
- Articles with unsourced statements from August 2010
- Drug
- Anabolic steroids