Methadone (oral): Difference between revisions
Gerald Chi (talk | contribs) m (Protected "Methadone" ([Edit=Allow only autoconfirmed users] (expires 19:00, 9 June 2014 (UTC)) [Move=Allow only autoconfirmed users] (expires 19:00, 9 June 2014 (UTC)))) |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag={{chetan}} | ||
|genericName=Methadone | |||
| | |aOrAn=an | ||
| | |drugClass=analgesic opioid | ||
| | |indicationType=treatment | ||
|indication=drug detoxification - opioid abuse, opioid abuse, maintenance therapy, pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time, pain (moderate to severe), not responsive to non-narcotic analgesics. | |||
| | |hasBlackBoxWarning=Yes | ||
| | |adverseReactions=cardiovascular: [[hypotension]], endocrine metabolic: [[diaphoresis]], [[gastrointestinal]]: [[constipation]], [[nausea]], [[vomiting]], [[neurologic]]: [[asthenia]], [[dizziness]], [[lightheadedness]], [[sedated]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">Conditions For Distribution And Use Of Methadone Products</span></b> | |||
|blackBoxWarningBody={{clr}} | |||
* Methadone products when used for the treatment of narcotic addiction in detoxification or maintenance programs, shall be dispensed only by approved hospital pharmacies, approved community pharmacies and by maintenance programs approved by the food and drug administration and the designated state authority. | |||
* Approved maintenance programs shall dispense and use methadone in oral form only and according to the treatment requirements stipulated in the federal methadone regulations (21 CFR 291.505). | |||
*Failure to abide by the requirements in these regulations may result in criminal prosecution, seizure of the drug supply, revocation of the program approval and injunction precluding operation of the program. | |||
| | |fdaLIADAdult=* Pharmacokinetic properties and high inter patient variability in absorption, metabolism, and relative analgesic potency of methadone necessitates a cautious an individualized approach to prescribing; careful dose initiation and titration are necessary. | ||
| | * Do not abruptly discontinue. | ||
* Conversion from oral methadone to parenteral methadone; initially use a 2:1 dose ratio (eg, 10 milligrams oral methadone to 5 milligrams parenteral methadone. | |||
| | * Conversion from other opioids to methadone; conversion ratios may overestimate dose of methadone; use cautiously. | ||
* Drug detoxification - Opioid abuse: initial, 20 to 30 mg orally administered when there are no signs of sedation or intoxication, and patient shows signs of withdrawal; Max initial dose 30 mg. | |||
| | * Drug detoxification - Opioid abuse: maintenance, additional 5 to 10 mg can be given 2 to 4 hours later if needed; adjust dose cautiously over the first week based upon control of withdrawal 2 to 4 hours post dose; usual total daily dose is 40 mg/day. | ||
| | * Drug detoxification - Opioid abuse: short-term detoxification, titrate to about 40 mg/day in divided doses; keep on stable dose for 2 to 3 days then decrease in 1 to 2 day intervals according to response. | ||
| | * Drug detoxification - Opioid abuse: maintenance treatment, titrate to a dose that prevents opioid withdrawal for 24 hours; generally 80 to 120 mg/day. | ||
| | * Drug detoxification - Opioid abuse: (unable to take oral medication) parenteral methadone may be used. | ||
* Opioid abuse, maintenance therapy: maintenance doses must be individualized; methadone doses should be titrated to a dose where symptoms are prevented for 24 hours; usual maintenance doses range from 80 to 120 mg/day. | |||
* Opioid abuse, maintenance therapy: (unable to take oral medication) parenteral methadone may be used. | |||
| | * Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: (opioid naive) initial, no more than 2.5 mg to 10 mg orally every 8 to 12 hours. | ||
* Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: (titration and maintenance) titrate slowly to effect; dose adjustment every 1 to 2 days; immediate-release rescue medication may be needed during titration. | |||
* Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: (discontinuation) do not discontinue abruptly; gradual downward titration every 2 to 4 days. | |||
* Pain (moderate to severe), not responsive to non-narcotic analgesics: (opioid naive) initial, 2.5 mg to 10 mg IV/IM/SubQ every 8 to 12 hours; titrate slowly based on individual response (manufacturer dosing). | |||
* Pain (moderate to severe), not responsive to non-narcotic analgesics: opioid naive patients: (initial) 2.5 mg ORAL/IV/IM/SubQ every 8 hour (guideline dosing). | |||
|offLabelAdultGuideSupport=* Neuropathic pain. | |||
|offLabelAdultNoGuideSupport=* There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Methadone in adult patients. | |||
|fdaLIADPed=* Safety and efficacy not established in pediatric patients younger than 18 years of age. | |||
* Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: 0.1 to 0.2 mg/kg Orally every 6 hour as needed; Max 10 mg/dose. | |||
* Pain (Moderate to Severe), Not responsive to non-narcotic analgesics: 0.1 to 0.2 mg/kg Orally every 6 hours as needed; MAX 10 mg/dose. | |||
|offLabelPedGuideSupport=* There is limited information about <i>Off-Label Guideline-Supported Use</i> of Methadone in pediatric patients. | |||
|offLabelPedNoGuideSupport=* There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Methadone in pediatric patients. | |||
|contraindications=* [[Hypersensitivity]] to methadone. | |||
|warnings=* Methadone hydrochloride powder is for oral administration only and is used in the preparation of a liquid by dissolving the powder in an appropriate vehicle. This preparation must not be injected. It is recommended that the methadone hydrochloride liquid preparation, if dispensed, be packaged in child-resistant containers and kept out of the reach of children to prevent accidental ingestion. | |||
* Methadone hydrochloride, a narcotic, is a Schedule II controlled substance under the Federal Controlled Substances Act. Appropriate security measures should be taken to safeguard stocks of methadone against diversion. | |||
|clinicalTrials=* Heroin Withdrawal: | |||
:* During the induction phase of methadone maintenance treatment, patients are being withdrawn from heroin and may therefore show typical withdrawal symptoms, which should be differentiated from methadone-induced side-effects. They may exhibit some or all of the following symptoms associated with acute withdrawal from [[heroin]] or other [[opiates]]: [[lacrimation]], [[rhinorrhea]], [[sneezing]], [[yawning]], excessive [[perspiration]], [[gooseflesh]], fever, chilliness alternating with flushing, restlessness, irritability, “sleepy yen,” [[weakness]], [[anxiety]], depression, [[dilated pupils]], [[tremors]], [[tachycardia]], [[abdominal cramps]], body aches, involuntary [[twitching]] and kicking movements, [[anorexia]], [[nausea]], [[vomiting]], [[diarrhea]], [[intestinal spasms]], and [[weight loss]]. | |||
* Initial Administration: | |||
:* Initially, the dosage of methadone should be carefully titrated to the individual. Induction too rapid for the patient’s sensitivity is more likely to produce the following effects. | |||
:* The major hazards of methadone, as of other narcotic analgesics, are respiratory depression and, to a lesser degree, circulatory depression. [[respiratory arrest]], [[shock]], and [[cardiac arrest]] have occurred. | |||
:* The most frequently observed adverse reactions include [[lightheadedness]], [[dizziness]], [[sedation]], [[nausea]], [[vomiting]], and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain. In such individuals, lower doses are advisable. Some adverse reactions may be alleviated in the ambulatory patient if he lies down. | |||
* Other adverse reactions include the following: | |||
:* Central Nervous System – [[euphoria]], [[dysphoria]], weakness, headache, insomnia, agitation, [[disorientation]], and visual disturbances. | |||
:* Gastro-Intestinal – Dry mouth, anorexia, [[constipation]], and [[biliary tract spasm]]. | |||
:* Cardiovascular – Flushing of the face, [[bradycardia]], [[palpitation]], [[faintness]], and syncope. | |||
:* Genito-Urinary – Urinary retention or hesitancy, antidiuretic effect, and reduced libido and/or potency. | |||
:* Allergic – [[pruritus]], [[urticaria]], other skin rashes, edema, and, rarely, hemorrhagic [[urticaria]]. | |||
* Maintenance on a Stabilized Dose: | |||
:* During prolonged administration of methadone, as in a methadone maintenance treatment program, there is a gradual, yet progressive, disappearance of side-effects over a period of several weeks. However, constipation and sweating often persist. | |||
|drugInteractions=* Interaction with [[Pentazocine]]: | |||
:* Patients who are addicted to heroin or who are on the methadone maintenance program may experience withdrawal symptoms when given pentazocine. | |||
* Interaction with [[Rifampin]]: | |||
:* The concurrent administration of [[rifampin]] may possibly reduce the blood concentration of methadone to a degree sufficient to produce withdrawal symptoms. The mechanism by which rifampin may decrease blood concentrations of methadone is not fully understood, although enhanced microsomal drug-metabolized enzymes may influence drug disposition. | |||
The | * Acute Abdominal Conditions: | ||
:* The administration of methadone or other narcotics may obscure the diagnosis or clinical course patients with acute abdominal conditions. | |||
* Interaction with Monoamine Oxidase (MAO) Inhibitors: | |||
:* Therapeutic doses of meperidine have precipitated severe reactions patients concurrently receiving monoamine oxidase inhibitors or those who have received such agents with fourteen days. Similar reactions thus far have not been reported with methadone; but if the use of methadone is necessary in such patients, a sensitivity test should be performed in which repeated small incremental doses are administered over the course of several hours while the patient’s condition and vital signs are under careful observation. | |||
|useInPregnancyFDA=* Caution shall be taken in the maintenance treatment of pregnant patients. Dosage levels shall be kept as low as possible if continued methadone treatment is deemed necessary. It is the responsibility of the program sponsor to assure that each female patient be fully informed concerning the possible risks to a pregnant woman or her unborn child from the use of methadone. | |||
|overdose=* Symptoms: | |||
:* Serious overdosage of methadone is characterized by [[respiratory depression]] (a decrease in respiratory rate and/or [[tidal volume]], [[Cheyne-Stokes respiration]], [[cyanosis]]), extreme [[somnolence]] progressing to [[stupor]] or [[coma]], maximally constricted [[pupils]], [[skeletal-muscle flaccidity]], [[cold and clammy skin]], and, sometimes, [[bradycardia]] and [[hypotension]]. In severe overdosage, particularly by the intravenous route, [[apnea]], [[circulatory collapse]], [[cardiac arrest]], and death may occur. | |||
* Treatment: | |||
:* Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. If a non-tolerant person, especially a child, takes a large dose of methadone, effective narcotic antagonists are available to counter-act the potentially lethal respiratory depression. The physician must remember, however, that methadone is a long-acting depressant (thirty-six to forty-eight hours), whereas the antagonists act for much shorter periods (one to three hours). The patient must, therefore, be monitored continuously for recurrence of respiratory depression and treated repeatedly with the narcotic antagonist as needed. If the diagnosis is correct and respiratory depression is due only to overdosage of methadone, the use of respiratory stimulants is not indicated. | |||
:* An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Intravenously administered narcotic antagonists, naloxone hydrochloride, nalorphine hydrochloride, or levallorphan tartrate are the drugs of choice to reverse signs of intoxication. These agents should be given repeatedly until the patient's status remains satisfactory. The hazard that the narcotic antagonist will further depress respiration is less likely with the use of naloxone. | |||
:* Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. | |||
Note: In an individual physically dependent on narcotics, the administration of the usual dose of a narcotic antagonist will precipitate an acute withdrawal syndrome. the severity of this syndrome will depend on the degree of physical dependence and the dose of the antagonist administered. the use of a narcotic antagonist in such a person should be avoided if possible. if it must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and by titration with smaller than usual doses of the antagonist. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 420419989 | |||
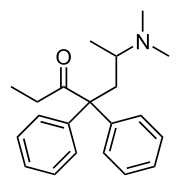
| IUPAC_name = (''RS'')-6-(Dimethylamino)-4,4-diphenylheptan-3-one | |||
| image = Methadone wiki1.png | |||
| width = 180px | |||
| image2 = Methadonewiki2.gif | |||
| width2 = 280px | |||
| imagename = 1 : 1 Mixture ([[racemate]]) | |||
| drug_name = Methadone | |||
=== | <!--Clinical data--> | ||
A | | tradename = Dolophine | ||
| Drugs.com = {{drugs.com|CDI|methadone}} | |||
| MedlinePlus = a682134 | |||
| pregnancy_AU = C | |||
| pregnancy_US = C | |||
| legal_AU = Schedule 8 | |||
| legal_US = Schedule II | |||
| legal_UK = Class A | |||
| dependency_liability = <!--need a reliable [[WP:MEDRS]]-compliant source to include this information--> | |||
| routes_of_administration = Oral, intravenous, insufflation, sublingual, rectal | |||
== | <!--Pharmacokinetic data--> | ||
| bioavailability = 41-99% (oral)<ref name = acta08>{{cite journal|last=Fredheim|first=OM|coauthors=Moksnes, K; Borchgrevink, PC; Kaasa, S; Dale, O|title=Clinical pharmacology of methadone for pain.|journal=Acta Anaesthesiologica Scandinavica|date=August 2008|volume=52|issue=7|pages=879–89|doi=10.1111/j.1399-6576.2008.01597.x|pmid=18331375}}</ref> | |||
=== | | protein_binding = 85-90%<ref name = acta08/> | ||
| metabolism = [[Hepatic]] ([[CYP3A4]], [[CYP2B6]] and [[CYP2D6]]-mediated)<ref name = acta08/><ref name = PMJ04>{{cite journal|last=Brown|first=R|coauthors=Kraus, C; Fleming, M; Reddy, S|title=Methadone: applied pharmacology and use as adjunctive treatment in chronic pain.|journal=Postgraduate Medical Journal|date=November 2004|volume=80|issue=949|pages=654–9|doi=10.1136/pgmj.2004.022988|pmid=15537850|url=http://pmj.bmj.com/content/80/949/654.full.pdf|format=PDF|pmc=1743125}}</ref> | |||
| elimination_half-life = 7-65 hours<ref name = PMJ04/> | |||
| excretion = Urine, faeces<ref name = PMJ04/> | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 76-99-3 | |||
| ATC_prefix = N02 | |||
| ATC_suffix = AC52 | |||
| ATC_supplemental = {{ATC|N07|BC02}} | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 6807 | |||
| PubChem = 4095 | |||
| IUPHAR_ligand = 1605 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00333 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3953 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = UC6VBE7V1Z | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08195 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 651 | |||
<!--Chemical data--> | |||
| C=21 | H=27 | N=1 | O=1 | |||
| molecular_weight = 309.445 g/mol | |||
| smiles = CCC(C(C1=CC=CC=C1)(C2=CC=CC=C2)CC(N(C)C)C)=O | |||
| InChI = 1/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = USSIQXCVUWKGNF-UHFFFAOYSA-N | |||
}} | |||
| | |mechAction=* Methadone hydrochloride is a synthetic narcotic analgesic with multiple actions quantitatively similar to those of morphine, the most prominent of which involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value are analgesia and sedation, detoxification or maintenance in narcotic addiction. The methadone abstinence syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe. | ||
| | * When administered orally, methadone is approximately one-half as potent as when given parenterally. Oral administration results in a delay of the onset, a lowering of the peak, and an increase in the duration of analgesic effect. | ||
|structure=* Methadone Hydrochloride is a white powder. It is chemically named 6-dimethylamino-4, 4-diphenyl-3-heptanone hydrochloride. | |||
| | |howSupplied=* Methadone Hydrochloride is supplied in containers of 50 gm., 100 gm., 500 gm., and 1 kilogram. | ||
* Preserve in tight, light-resistant containers. Store at controlled room temperature (15° to 30°C.) (59° to 86°F.) | |||
MALLINCKRODT | |||
MALLINCKRODT INC. | |||
St. Louis, Missouri 63134 | |||
July 1998 | |||
1274111 | |||
Printed in U.S.A. | |||
|alcohol=* Alcohol-Methadone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
{{PillImage | |||
|fileName=Pill_Image.jpeg | |||
|drugName=Methadone | |||
|NDC=0406-1510-56 | |||
|drugAuthor=Mallinckrodt, Inc. | |||
|ingredients=Lactose monohydrate, magnesium stearate, cellulose, microcrystalline, silicon dioxide | |||
|pillImprint=57;55;M | |||
|dosageValue=5 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=Rectangular | |||
|pillSize=9.00 | |||
|pillScore=2 | |||
}} | |||
{{LabelImage | |||
|fileName=Methadone label.png | |||
}} | |||
= | |||
== | |||
= | |||
{{ | |||
Latest revision as of 18:36, 15 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Conditions For Distribution And Use Of Methadone Products
See full prescribing information for complete Boxed Warning.
|
Overview
Methadone (oral) is an analgesic opioid that is FDA approved for the treatment of drug detoxification - opioid abuse, opioid abuse, maintenance therapy, pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time, pain (moderate to severe), not responsive to non-narcotic analgesics.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cardiovascular: hypotension, endocrine metabolic: diaphoresis, gastrointestinal: constipation, nausea, vomiting, neurologic: asthenia, dizziness, lightheadedness, sedated.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Pharmacokinetic properties and high inter patient variability in absorption, metabolism, and relative analgesic potency of methadone necessitates a cautious an individualized approach to prescribing; careful dose initiation and titration are necessary.
- Do not abruptly discontinue.
- Conversion from oral methadone to parenteral methadone; initially use a 2:1 dose ratio (eg, 10 milligrams oral methadone to 5 milligrams parenteral methadone.
- Conversion from other opioids to methadone; conversion ratios may overestimate dose of methadone; use cautiously.
- Drug detoxification - Opioid abuse: initial, 20 to 30 mg orally administered when there are no signs of sedation or intoxication, and patient shows signs of withdrawal; Max initial dose 30 mg.
- Drug detoxification - Opioid abuse: maintenance, additional 5 to 10 mg can be given 2 to 4 hours later if needed; adjust dose cautiously over the first week based upon control of withdrawal 2 to 4 hours post dose; usual total daily dose is 40 mg/day.
- Drug detoxification - Opioid abuse: short-term detoxification, titrate to about 40 mg/day in divided doses; keep on stable dose for 2 to 3 days then decrease in 1 to 2 day intervals according to response.
- Drug detoxification - Opioid abuse: maintenance treatment, titrate to a dose that prevents opioid withdrawal for 24 hours; generally 80 to 120 mg/day.
- Drug detoxification - Opioid abuse: (unable to take oral medication) parenteral methadone may be used.
- Opioid abuse, maintenance therapy: maintenance doses must be individualized; methadone doses should be titrated to a dose where symptoms are prevented for 24 hours; usual maintenance doses range from 80 to 120 mg/day.
- Opioid abuse, maintenance therapy: (unable to take oral medication) parenteral methadone may be used.
- Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: (opioid naive) initial, no more than 2.5 mg to 10 mg orally every 8 to 12 hours.
- Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: (titration and maintenance) titrate slowly to effect; dose adjustment every 1 to 2 days; immediate-release rescue medication may be needed during titration.
- Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: (discontinuation) do not discontinue abruptly; gradual downward titration every 2 to 4 days.
- Pain (moderate to severe), not responsive to non-narcotic analgesics: (opioid naive) initial, 2.5 mg to 10 mg IV/IM/SubQ every 8 to 12 hours; titrate slowly based on individual response (manufacturer dosing).
- Pain (moderate to severe), not responsive to non-narcotic analgesics: opioid naive patients: (initial) 2.5 mg ORAL/IV/IM/SubQ every 8 hour (guideline dosing).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Neuropathic pain.
Non–Guideline-Supported Use
- There is limited information about Off-Label Non–Guideline-Supported Use of Methadone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy not established in pediatric patients younger than 18 years of age.
- Pain, chronic (moderate to severe), in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time: 0.1 to 0.2 mg/kg Orally every 6 hour as needed; Max 10 mg/dose.
- Pain (Moderate to Severe), Not responsive to non-narcotic analgesics: 0.1 to 0.2 mg/kg Orally every 6 hours as needed; MAX 10 mg/dose.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information about Off-Label Guideline-Supported Use of Methadone in pediatric patients.
Non–Guideline-Supported Use
- There is limited information about Off-Label Non–Guideline-Supported Use of Methadone in pediatric patients.
Contraindications
- Hypersensitivity to methadone.
Warnings
|
Conditions For Distribution And Use Of Methadone Products
See full prescribing information for complete Boxed Warning.
|
- Methadone hydrochloride powder is for oral administration only and is used in the preparation of a liquid by dissolving the powder in an appropriate vehicle. This preparation must not be injected. It is recommended that the methadone hydrochloride liquid preparation, if dispensed, be packaged in child-resistant containers and kept out of the reach of children to prevent accidental ingestion.
- Methadone hydrochloride, a narcotic, is a Schedule II controlled substance under the Federal Controlled Substances Act. Appropriate security measures should be taken to safeguard stocks of methadone against diversion.
Adverse Reactions
Clinical Trials Experience
- Heroin Withdrawal:
- During the induction phase of methadone maintenance treatment, patients are being withdrawn from heroin and may therefore show typical withdrawal symptoms, which should be differentiated from methadone-induced side-effects. They may exhibit some or all of the following symptoms associated with acute withdrawal from heroin or other opiates: lacrimation, rhinorrhea, sneezing, yawning, excessive perspiration, gooseflesh, fever, chilliness alternating with flushing, restlessness, irritability, “sleepy yen,” weakness, anxiety, depression, dilated pupils, tremors, tachycardia, abdominal cramps, body aches, involuntary twitching and kicking movements, anorexia, nausea, vomiting, diarrhea, intestinal spasms, and weight loss.
- Initial Administration:
- Initially, the dosage of methadone should be carefully titrated to the individual. Induction too rapid for the patient’s sensitivity is more likely to produce the following effects.
- The major hazards of methadone, as of other narcotic analgesics, are respiratory depression and, to a lesser degree, circulatory depression. respiratory arrest, shock, and cardiac arrest have occurred.
- The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain. In such individuals, lower doses are advisable. Some adverse reactions may be alleviated in the ambulatory patient if he lies down.
- Other adverse reactions include the following:
- Central Nervous System – euphoria, dysphoria, weakness, headache, insomnia, agitation, disorientation, and visual disturbances.
- Gastro-Intestinal – Dry mouth, anorexia, constipation, and biliary tract spasm.
- Cardiovascular – Flushing of the face, bradycardia, palpitation, faintness, and syncope.
- Genito-Urinary – Urinary retention or hesitancy, antidiuretic effect, and reduced libido and/or potency.
- Allergic – pruritus, urticaria, other skin rashes, edema, and, rarely, hemorrhagic urticaria.
- Maintenance on a Stabilized Dose:
- During prolonged administration of methadone, as in a methadone maintenance treatment program, there is a gradual, yet progressive, disappearance of side-effects over a period of several weeks. However, constipation and sweating often persist.
Postmarketing Experience
There is limited information regarding Methadone (oral) Postmarketing Experience in the drug label.
Drug Interactions
- Interaction with Pentazocine:
- Patients who are addicted to heroin or who are on the methadone maintenance program may experience withdrawal symptoms when given pentazocine.
- Interaction with Rifampin:
- The concurrent administration of rifampin may possibly reduce the blood concentration of methadone to a degree sufficient to produce withdrawal symptoms. The mechanism by which rifampin may decrease blood concentrations of methadone is not fully understood, although enhanced microsomal drug-metabolized enzymes may influence drug disposition.
- Acute Abdominal Conditions:
- The administration of methadone or other narcotics may obscure the diagnosis or clinical course patients with acute abdominal conditions.
- Interaction with Monoamine Oxidase (MAO) Inhibitors:
- Therapeutic doses of meperidine have precipitated severe reactions patients concurrently receiving monoamine oxidase inhibitors or those who have received such agents with fourteen days. Similar reactions thus far have not been reported with methadone; but if the use of methadone is necessary in such patients, a sensitivity test should be performed in which repeated small incremental doses are administered over the course of several hours while the patient’s condition and vital signs are under careful observation.
Use in Specific Populations
Pregnancy
- Caution shall be taken in the maintenance treatment of pregnant patients. Dosage levels shall be kept as low as possible if continued methadone treatment is deemed necessary. It is the responsibility of the program sponsor to assure that each female patient be fully informed concerning the possible risks to a pregnant woman or her unborn child from the use of methadone.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methadone (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methadone (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Methadone (oral) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Methadone (oral) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Methadone (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Methadone (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methadone (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methadone (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methadone (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methadone (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methadone (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Methadone (oral) Administration in the drug label.
Monitoring
There is limited information regarding Methadone (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Methadone (oral) and IV administrations.
Overdosage
- Symptoms:
- Serious overdosage of methadone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, maximally constricted pupils, skeletal-muscle flaccidity, cold and clammy skin, and, sometimes, bradycardia and hypotension. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.
- Treatment:
- Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. If a non-tolerant person, especially a child, takes a large dose of methadone, effective narcotic antagonists are available to counter-act the potentially lethal respiratory depression. The physician must remember, however, that methadone is a long-acting depressant (thirty-six to forty-eight hours), whereas the antagonists act for much shorter periods (one to three hours). The patient must, therefore, be monitored continuously for recurrence of respiratory depression and treated repeatedly with the narcotic antagonist as needed. If the diagnosis is correct and respiratory depression is due only to overdosage of methadone, the use of respiratory stimulants is not indicated.
- An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Intravenously administered narcotic antagonists, naloxone hydrochloride, nalorphine hydrochloride, or levallorphan tartrate are the drugs of choice to reverse signs of intoxication. These agents should be given repeatedly until the patient's status remains satisfactory. The hazard that the narcotic antagonist will further depress respiration is less likely with the use of naloxone.
- Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
Note: In an individual physically dependent on narcotics, the administration of the usual dose of a narcotic antagonist will precipitate an acute withdrawal syndrome. the severity of this syndrome will depend on the degree of physical dependence and the dose of the antagonist administered. the use of a narcotic antagonist in such a person should be avoided if possible. if it must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and by titration with smaller than usual doses of the antagonist.
Pharmacology
| |
| |
1 : 1 Mixture (racemate)Methadone
| |
| Systematic (IUPAC) name | |
| (RS)-6-(Dimethylamino)-4,4-diphenylheptan-3-one | |
| Identifiers | |
| CAS number | |
| ATC code | N02 N07BC02 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 309.445 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 41-99% (oral)[1] |
| Metabolism | Hepatic (CYP3A4, CYP2B6 and CYP2D6-mediated)[1][2] |
| Half life | 7-65 hours[2] |
| Excretion | Urine, faeces[2] |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral, intravenous, insufflation, sublingual, rectal |
Mechanism of Action
- Methadone hydrochloride is a synthetic narcotic analgesic with multiple actions quantitatively similar to those of morphine, the most prominent of which involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value are analgesia and sedation, detoxification or maintenance in narcotic addiction. The methadone abstinence syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe.
- When administered orally, methadone is approximately one-half as potent as when given parenterally. Oral administration results in a delay of the onset, a lowering of the peak, and an increase in the duration of analgesic effect.
Structure
- Methadone Hydrochloride is a white powder. It is chemically named 6-dimethylamino-4, 4-diphenyl-3-heptanone hydrochloride.
Pharmacodynamics
There is limited information regarding Methadone (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Methadone (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Methadone (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Methadone (oral) Clinical Studies in the drug label.
How Supplied
- Methadone Hydrochloride is supplied in containers of 50 gm., 100 gm., 500 gm., and 1 kilogram.
- Preserve in tight, light-resistant containers. Store at controlled room temperature (15° to 30°C.) (59° to 86°F.)
MALLINCKRODT MALLINCKRODT INC. St. Louis, Missouri 63134 July 1998 1274111 Printed in U.S.A.
Storage
There is limited information regarding Methadone (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Methadone (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Methadone (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Methadone (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Methadone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Methadone (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Methadone (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Fredheim, OM (August 2008). "Clinical pharmacology of methadone for pain". Acta Anaesthesiologica Scandinavica. 52 (7): 879–89. doi:10.1111/j.1399-6576.2008.01597.x. PMID 18331375. Unknown parameter
|coauthors=ignored (help) - ↑ 2.0 2.1 2.2 Brown, R (November 2004). "Methadone: applied pharmacology and use as adjunctive treatment in chronic pain" (PDF). Postgraduate Medical Journal. 80 (949): 654–9. doi:10.1136/pgmj.2004.022988. PMC 1743125. PMID 15537850. Unknown parameter
|coauthors=ignored (help)
{{#subobject:
|Page Name=Methadone (oral) |Pill Name=Pill_Image.jpeg |Drug Name=Methadone |Pill Ingred=Lactose monohydrate, magnesium stearate, cellulose, microcrystalline, silicon dioxide|+sep=; |Pill Imprint=57;55;M |Pill Dosage=5 mg |Pill Color=White|+sep=; |Pill Shape=Rectangular |Pill Size (mm)=9.00 |Pill Scoring=2 |Pill Image= |Drug Author=Mallinckrodt, Inc. |NDC=0406-1510-56
}}
{{#subobject:
|Label Page=Methadone (oral) |Label Name=Methadone label.png
}}