Metabolism
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Metabolism is the complete set of chemical reactions that occur in living cells. These processes are the basis of life, allowing cells to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories. Catabolism, which yields energy, an example being is the breakdown of food in cellular respiration. Anabolism, on the other hand, uses this energy to construct components of cells such as proteins and nucleic acids.
The chemical reactions of metabolism are organised into metabolic pathways, in which one chemical is transformed into another by a sequence of enzymes. Enzymes are crucial to metabolism because they allow cells to drive desirable but thermodynamically unfavorable reactions by coupling them to favorable ones. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or signals from other cells.
The metabolism of an organism determines which substances it will find nutritious and which it will find poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals.[1] The speed of metabolism, the metabolic rate, also influences how much food an organism will require.
A striking feature of metabolism is the similarity of the basic metabolic pathways between even vastly different species. For example, the set of chemical intermediates in the citric acid cycle are found universally, among living cells as diverse as the unicellular bacteria Escherichia coli and huge multicellular organisms like elephants.[2] This shared metabolic structure is most likely the result of the high efficiency of these pathways, and of their early appearance in evolutionary history.[3][4]
Key biochemicals
Most of the structures that make up animals, plants and microbes are made from three basic classes of molecule: amino acids, carbohydrates and lipids (often called fats). As these molecules are vital for life, metabolism focuses on making these molecules, in the construction of cells and tissues, or breaking them down and using them as a source of energy, in the digestion and use of food. Many important biochemicals can be joined together to make polymers such as DNA and proteins. These macromolecules are essential parts of all living organisms. Some of the most common biological polymers are listed in the table below.
| Type of molecule | Name of monomer forms | Name of polymer forms | Examples of polymer forms |
|---|---|---|---|
| Amino acids | Amino acids | Proteins (also called polypeptides) | Fibrous proteins and globular proteins |
| Carbohydrates | Monosaccharides | Polysaccharides | Starch, glycogen and cellulose |
| Nucleic acids | Nucleotides | Polynucleotides | DNA and RNA |
Amino acids and proteins
Proteins are made of amino acids arranged in a linear chain and joined together by peptide bonds. Many proteins are the enzymes that catalyze the chemical reactions in metabolism. Other proteins have structural or mechanical functions, such as the proteins in the cytoskeleton that form a system of scaffolding to maintain cell shape.[5] Proteins are also important in cell signaling, immune responses, cell adhesion, active transport across membranes and the cell cycle.[6]
Lipids
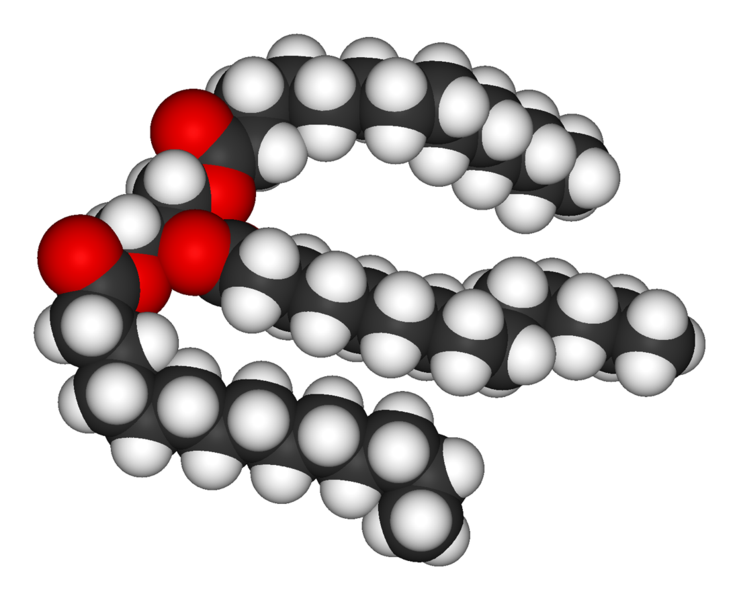
Lipids are the most diverse group of biochemicals. Their main structural uses are as part of biological membranes such as the cell membrane, or as a source of energy.[6] Lipids are usually defined as hydrophobic or amphipathic biological molecules that will dissolve in organic solvents such as benzene or chloroform.[7] The fats are a large group of compounds that contain fatty acids and glycerol; a glycerol molecule attached to three fatty acid esters is a triacylglyceride.[8] Several variations on this basic structure exist, including alternate backbones such as sphingosine in the sphingolipids, and hydrophilic groups such as phosphate in phospholipids. Steroids such as cholesterol are another major class of lipids that are made in cells.[9]
Carbohydrates
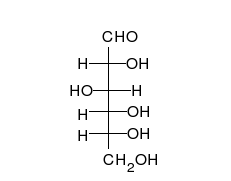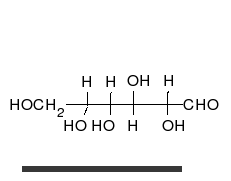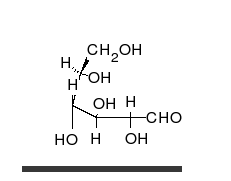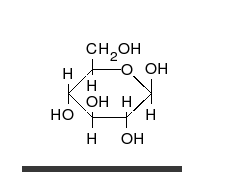
Carbohydrates are straight-chain aldehydes or ketones with many hydroxyl groups that can exist as straight chains or rings. Carbohydrates are the most abundant biological molecules, and fill numerous roles, such as the storage and transport of energy (starch, glycogen) and structural components (cellulose in plants, chitin in animals).[6] The basic carbohydrate units are called monosaccharides and include galactose, fructose, and most importantly glucose. Monosaccharides can be linked together to form polysaccharides in almost limitless ways.[10]
Nucleotides
The polymers DNA and RNA are long chains of nucleotides. These molecules are critical for the storage and use of genetic information, through the processes of transcription and protein biosynthesis.[6] This information is protected by DNA repair mechanisms and propagated through DNA replication. A few viruses have an RNA genome, for example HIV, which uses reverse transcription to create a DNA template from its viral RNA genome.[11] RNA in ribozymes such as spliceosomes and ribosomes is similar to enzymes as it can catalyze chemical reactions. Individual nucleosides are made by attaching a nucleobase to a ribose sugar. These bases are heterocyclic rings containing nitrogen, classified as purines or pyrimidines. Nucleotides also act as coenzymes in metabolic group transfer reactions.[12]
Coenzymes
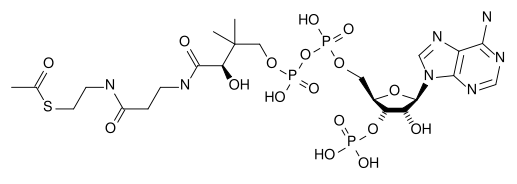
Metabolism involves a vast array of chemical reactions, but most fall under a few basic types of reactions that involve the transfer of functional groups.[13] This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions.[12] These group-transfer intermediates are called coenzymes. Each class of group-transfer reaction is carried out by a particular coenzyme, which is the substrate for a set of enzymes that produce it, and a set of enzymes that consume it. These coenzymes are therefore continuously being made, consumed and then recycled.[14]
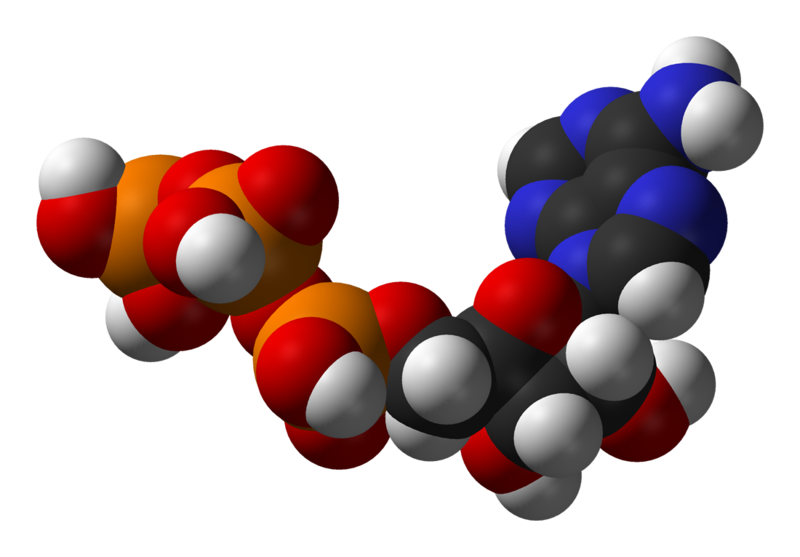
The most central coenzyme is adenosine triphosphate (ATP), the universal energy currency of cells. This nucleotide is used to transfer chemical energy between different chemical reactions. There is only a small amount of ATP in cells, but as it is continuously regenerated, the human body can use about its own weight in ATP per day.[14] ATP acts as a bridge between catabolism and anabolism, with catabolic reactions generating ATP and anabolic reactions consuming it. It also serves as a carrier of phosphate groups in phosphorylation reactions.
A vitamin is an organic compound needed in small quantities that cannot be made in the cells. In human nutrition, most vitamins function as coenzymes after modification; for example, all water-soluble vitamins are phosphorylated or are coupled to nucleotides when they are used in cells.[15] Nicotinamide adenine dinucleotide (NADH), a derivative of vitamin B3 (niacin), is an important coenzyme that acts as a hydrogen acceptor. Hundreds of separate types of dehydrogenases remove electrons from their substrates and reduce NAD+ into NADH. This reduced form of the coenzyme is then a substrate for any of the reductases in the cell that need to reduce their substrates.[16] Nicotinamide adenine dinucleotide exists in two related forms in the cell, NADH and NADPH. The NAD+/NADH form is more important in catabolic reactions, while NADP+/NADPH is used in anabolic reactions.
Minerals and cofactors
Inorganic elements play critical roles in metabolism; some are abundant (e.g. sodium and potassium) while others function at minute concentrations. About 99% of mammals' mass are the elements carbon, nitrogen, calcium, sodium, chlorine, potassium, hydrogen, oxygen and sulfur.[17] The organic compounds (proteins, lipids and carbohydrates) contain the majority of the carbon and nitrogen and most of the oxygen and hydrogen is present as water.[17]
The abundant inorganic elements act as ionic electrolytes. The most important ions are sodium, potassium, calcium, magnesium, chloride, phosphate, and the organic ion bicarbonate. The maintenance of precise gradients across cell membranes maintains osmotic pressure and pH.[18] Ions are also critical for nerves and muscles, as action potentials in these tissues are produced by the exchange of electrolytes between the extracellular fluid and the cytosol.[19] Electrolytes enter and leave cells through proteins in the cell membrane called ion channels. For example, muscle contraction depends upon the movement of calcium, sodium and potassium through ion channels in the cell membrane and T-tubules.[20]
The transition metals are usually present as trace elements in organisms, with zinc and iron being most abundant.[21][22] These metals are used in some proteins as cofactors and are essential for the activity of enzymes such as catalase and oxygen-carrier proteins such as hemoglobin.[23] These cofactors are bound tightly to a specific protein; although enzyme cofactors can be modified during catalysis, cofactors always return to their original state after catalysis has taken place. The metal micronutrients are taken up into organisms by specific transporters and bound to storage proteins such as ferritin or metallothionein when not being used.[24][25]
Catabolism
Catabolism is the set of metabolic processes that release energy. These include breaking down and oxidising food molecules as well as reactions that trap the energy in sunlight. The purpose of these catabolic reactions is to provide the energy and components needed by anabolic reactions. The exact nature of these catabolic reactions differ from organism to organism, with organic molecules being used as a source of energy in organotrophs, while lithotrophs use inorganic substrates and phototrophs capture sunlight as chemical energy. However, all these different forms of metabolism depend on redox reactions that involve the transfer of electrons from reduced donor molecules such as organic molecules, water, ammonia, hydrogen sulfide or ferrous ions to acceptor molecules such as oxygen, nitrate or sulphate.[26] In animals these reactions involve complex organic molecules being broken down to simpler molecules, such as carbon dioxide and water. In photosynthetic organisms such as plants and cyanobacteria, these electron-transfer reactions do not release energy, but are used as a way of storing energy absorbed from sunlight.[6]
The most common set of catabolic reactions in animals can be separated into three main stages. In the first, large organic molecules such as proteins, polysaccharides or lipids are digested into their smaller components outside cells. Next, these smaller molecules are taken up by cells and converted to yet smaller molecules, usually acetyl coenzyme A (CoA), which releases some energy. Finally, the acetyl group on the CoA is oxidised to water and carbon dioxide in the citric acid cycle and electron transport chain, releasing the energy that is stored by reducing the coenzyme nicotinamide adenine dinucleotide (NAD+) into NADH.
Digestion
Macromolecules such as starch, cellulose or proteins cannot be rapidly taken up by cells and need to be broken into their smaller units before they can be used in cell metabolism. Several common classes of enzymes digest these polymers. These digestive enzymes include proteases that digest proteins into amino acids, as well as glycoside hydrolases that digest polysaccharides into monosaccharides.
Microbes simply secrete digestive enzymes into their surroundings,[27][28] while animals only secrete these enzymes from specialized cells in their guts.[29] The amino acids or sugars released by these extracellular enzymes are then pumped into cells by specific active transport proteins.[30][31]
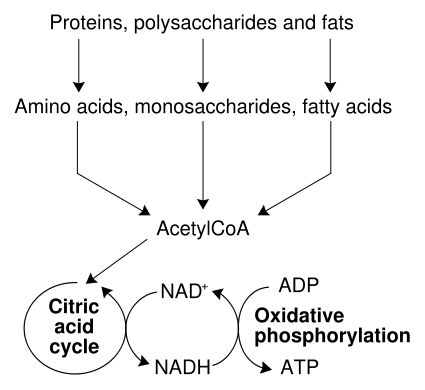
Energy from organic compounds
Carbohydrate catabolism is the breakdown of carbohydrates into smaller units. Carbohydrates are usually taken into cells once they have been digested into monosaccharides.[32] Once inside, the major route of breakdown is glycolysis, where sugars such as glucose and fructose are converted into pyruvate and some ATP is generated.[33] Pyruvate is an intermediate in several metabolic pathways, but the majority is converted to acetyl-CoA and fed into the citric acid cycle. Although some more ATP is generated in the citric acid cycle, the most important product is NADH, which is made from NAD+ as the acetyl-CoA is oxidized. This oxidation releases carbon dioxide as a waste product. In anaerobic conditions, glycolysis produces lactate, through the enzyme lactate dehydrogenase re-oxidizing NADH to NAD+ for re-use in glycolysis. An alternative route for glucose breakdown is the pentose phosphate pathway, which reduces the coenzyme NADPH and produces pentose sugars such as ribose, the sugar component of nucleic acids.
Fats are catabolised by hydrolysis to free fatty acids and glycerol. The glycerol enters glycolysis and the fatty acids are broken down by beta oxidation to release acetyl-CoA, which then is fed into the citric acid cycle. Fatty acids release more energy upon oxidation than carbohydrates because carbohydrates contain more oxygen in their structures.
Amino acids are either used to synthesize proteins and other biomolecules, or oxidized to urea and carbon dioxide as a source of energy.[34] The oxidation pathway starts with the removal of the amino group by a transaminase. The amino group is fed into the urea cycle, leaving a deaminated carbon skeleton in the form of a keto acid. Several of these keto acids are intermediates in the citric acid cycle, for example the deamination of glutamate forms α-ketoglutarate.[35] The glucogenic amino acids can also be converted into glucose, through gluconeogenesis (discussed below).[36]
Oxidative phosphorylation
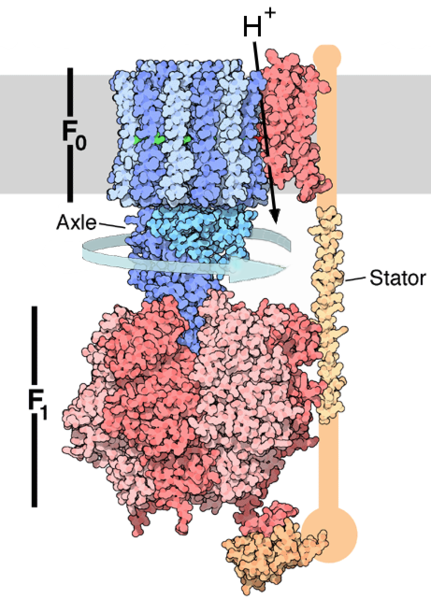
In oxidative phosphorylation, the electrons removed from food molecules in pathways such as the citric acid cycle are transferred to oxygen and the energy released used to make ATP. This is done in eukaryotes by a series of proteins in the membranes of mitochondria called the electron transport chain. In prokaryotes, these proteins are found in the cell's inner membrane.[37] These proteins use the energy released from passing electrons from reduced molecules like NADH onto oxygen to pump protons across a membrane.[38]
Pumping protons out of the mitochondria creates a proton concentration difference across the membrane and generates a electrochemical gradient.[39] This force drives protons back into the mitochondrion through the base of an enzyme called ATP synthase. The flow of protons makes the stalk subunit rotate, causing the active site of the synthase domain to change shape and phosphorylate adenosine diphosphate - turning it into ATP.[14]
Energy from inorganic compounds
Chemolithotrophy is a type of metabolism found in prokaryotes where energy is obtained from the oxidation of inorganic compounds. These organisms can use hydrogen,[40] reduced sulfur compounds (such as sulfide, hydrogen sulfide and thiosulfate),[41] ferrous iron (FeII)[42] or ammonia[43] as sources of reducing power and they gain energy from the oxidation of these compounds with electron acceptors such as oxygen or nitrite.[44] These microbial processes are important in global biogeochemical cycles such as acetogenesis, nitrification and denitrification and are critical for soil fertility.[45][46]
Energy from light
The energy in sunlight is captured by plants, cyanobacteria, purple bacteria, green sulfur bacteria and some protists. This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below. The energy capture and carbon fixation systems can however operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds.[47][48]
The capture of solar energy is a process that is similar in principle to oxidative phosphorylation, as it involves energy being stored as a proton concentration gradient and this proton motive force then driving ATP synthesis.[14] The electrons needed to drive this electron transport chain come from light-gathering proteins called photosynthetic reaction centres. These structures are classed into two types depending on the type of photosynthetic pigment present, with most photosynthetic bacteria only having one type of reaction center, while plants and cyanobacteria have two.[49]
In plants, photosystem II uses light energy to remove electrons from water, releasing oxygen as a waste product. The electrons then flow to the cytochrome b6f complex, which uses their energy to pump protons across the thylakoid membrane in the chloroplast.[50] These protons move back through the membrane as they drive the ATP synthase, as before. The electrons then flow through photosystem I and can then either be used to reduce the coenzyme NADP+, for use in the Calvin cycle which is discussed below, or recycled for further ATP generation.[51]
Anabolism
Anabolism is the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from small and simple precursors. Anabolism involves three basic stages. Firstly, the production of precursors such as amino acids, monosaccharides, isoprenoids and nucleotides, secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such as proteins, polysaccharides, lipids and nucleic acids.
Organisms differ in how many of the molecules in their cells they can construct for themselves. Autotrophs such as plants can construct the complex organic molecules in cells such as polysaccharides and proteins from simple molecules like carbon dioxide and water. Heterotrophs, on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from inorganic oxidation reactions.
Carbon fixation
Photosynthesis is the synthesis of glucose from sunlight, carbon dioxide (CO2) and water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by the photosynthetic reaction centres, as described above, to convert CO2 into glycerate 3-phosphate, which can then be converted into glucose. This carbon-fixation reaction is carried out by the enzyme RuBisCO as part of the Calvin – Benson cycle.[52] Three types of photosynthesis occur in plants, C3 carbon fixation, C4 carbon fixation and CAM photosynthesis. These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO2 directly, while C4 and CAM photosynthesis incorporate the CO2 into other compounds first, as adaptations to deal with intense sunlight and dry conditions.[53]
In photosynthetic prokaryotes the mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin – Benson cycle, a reversed citric acid cycle,[54] or the carboxylation of acetyl-CoA.[55][56] Prokaryotic chemoautotrophs also fix CO2 through the Calvin – Benson cycle, but use energy from inorganic compounds to drive the reaction.[57]
Carbohydrates and glycans
In carbohydrate anabolism, simple organic acids can be converted into monosaccharides such as glucose and then used to assemble polysaccharides such as starch. The generation of glucose from compounds like pyruvate, lactate, glycerol, glycerate 3-phosphate and amino acids is called gluconeogenesis. Gluconeogenesis converts pyruvate to glucose-6-phosphate through a series of intermediates, many of which are shared with glycolysis.[33] However, this pathway is not simply glycolysis run in reverse, as several steps are catalyzed by non-glycolytic enzymes. This is important as it allows the formation and breakdown of glucose to be regulated separately and prevents both pathways from running simultaneously in a futile cycle.[58][59]
Although fat is a common way of storing energy, in vertebrates such as humans the fatty acids in these stores cannot be converted to glucose through gluconeogenesis as these organisms cannot convert acetyl-CoA into pyruvate.[60] As a result, after long-term starvation, vertebrates need to produce ketone bodies from fatty acids to replace glucose in tissues such as the brain that cannot metabolize fatty acids.[61] In other organisms such as plants and bacteria, this metabolic problem is solved using the glyoxylate cycle, which bypasses the decarboxylation step in the citric acid cycle and allows the transformation of acetyl-CoA to oxaloacetate, where it can be used for the production of glucose.[62][60]
Polysaccharides and glycans are made by the sequential addition of monosaccharides by glycosyltransferase from a reactive sugar-phosphate donor such as uridine diphosphate glucose (UDP-glucose) to an acceptor hydroxyl group on the growing polysaccharide. As any of the hydroxyl groups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures.[63] The polysaccharides produced can have structural or metabolic functions themselves, or be transferred to lipids and proteins by enzymes called oligosaccharyltransferases.[64][65]
Fatty acids, isoprenoids and steroids
Fatty acids are made by fatty acid synthases that polymerize and reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the actyl group, reduce it to the alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional type I protein,[66] while in plant plastids and bacteria separate type II enzymes perform each step in the pathway.[67][68]
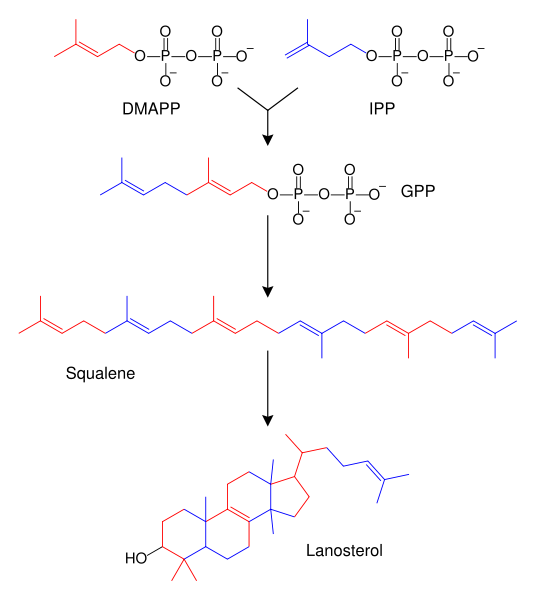
Terpenes and isoprenoids are a large class of lipids that include the carotenoids and form the largest class of plant natural products.[69] These compounds are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate.[70] These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA,[71] while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates.[72][70] One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol.[73] Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.[74][73]
Proteins
Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all twenty, but mammals can synthesize only the ten nonessential amino acids.[6] Thus, the essential amino acids must be obtained from food. All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen is provided by glutamate and glutamine. Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid.[75]
Amino acids are made into proteins by being joined together in a chain by peptide bonds. Each different protein has a unique sequence of amino acid residues: this is its primary structure. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to a transfer RNA molecule through an ester bond. This aminoacyl-tRNA precursor is produced in an ATP-dependent reaction carried out by an aminoacyl tRNA synthetase.[76] This aminoacyl-tRNA is then a substrate for the ribosome, which joins the amino acid onto the elongating protein chain, using the sequence information in a messenger RNA.[77]
Nucleotide synthesis and salvage
Nucleotides are made from amino acids, carbon dioxide and formic acid in pathways that require large amounts of metabolic energy.[78] Consequently, most organisms have efficient systems to salvage preformed nucleotides.[78][79] Purines are synthesized as nucleosides (bases attached to ribose). Both adenine and guanine are made from the precursor nucleoside inosine monophosphate, which is synthesized using atoms from the amino acids glycine, glutamine, and aspartic acid, as well as formate transferred from the coenzyme tetrahydrofolate. Pyrimidines, on the other hand, are synthesized from the base orotate, which is formed from glutamine and aspartate.[80]
Xenobiotics and redox metabolism
All organisms are constantly exposed to compounds that they cannot use as foods and would be harmful if they accumulated in cells, as they have no metabolic function. These potentially damaging compounds are called xenobiotics.[81] Xenobiotics such as synthetic drugs, natural poisons and antibiotics are detoxified by a set of xenobiotic-metabolizing enzymes. In humans, these include cytochrome P450 oxidases,[82] UDP-glucuronosyltransferasess,[83] and glutathione S-transferases.[84] This system of enzymes acts in three stages to firstly oxidize the xenobiotic (phase I) and then conjugate water-soluble groups onto the molecule (phase II). The modified water-soluble xenobiotic can then be pumped out of cells and in multicellular organisms may be further metabolized before being excreted (phase III). In ecology, these reactions are particularly important in microbial biodegradation of pollutants and the bioremediation of contaminated land and oil spills.[85] Many of these microbial reactions are shared with multicellular organisms, but due to their incredible diversity, microbes are able to deal with a far wider range of xenobiotics than multicellular organisms and can degrade even persistent organic pollutants such as organochloride compounds.[86]
A related problem for aerobic organisms is oxidative stress.[87] Here, processes including oxidative phosphorylation and the formation of disulfide bonds during protein folding produce reactive oxygen species such as hydrogen peroxide.[88] These damaging oxidants are removed by antioxidant metabolites such as glutathione and enzymes such as catalases and peroxidases.[89][90]
Thermodynamics of living organisms
Living organisms must obey the laws of thermodynamics, which describe the transfer of heat and work. The second law of thermodynamics states that in any closed system, the amount of entropy (disorder) will tend to increase. Although living organisms' amazing complexity appears to contradict this law, life is possible as all organisms are open systems that exchange matter and energy with their surroundings. Thus living systems are not in equilibrium, but instead are dissipative systems that maintain their state of high complexity by causing a larger increase in the entropy of their environments.[91] The metabolism of a cell achieves this by coupling the spontaneous processes of catabolism to the non-spontaneous processes of anabolism. In thermodynamic terms, metabolism maintains order by creating disorder.[92]
Regulation and control
As the environments of most organisms are constantly changing, the reactions of metabolism must be finely regulated to maintain a constant set of conditions within cells, a condition called homeostasis.[93][94] Metabolic regulation also allows organisms to respond to signals and interact actively with their environments.[95] Two closely-linked concepts are important for understanding how metabolic pathways are controlled. Firstly, the regulation of an enzyme in a pathway is how its activity is increased and decreased in response to signals. Secondly, the control exerted by this enzyme is the effect that these changes in its activity have on the overall rate of the pathway (the flux through the pathway).[96] For example, an enzyme may show large changes in activity (i.e. it is highly regulated) but if these changes have little effect on the flux of a metabolic pathway, then this enzyme is not involved in the control of the pathway.[97]

There are multiple levels of metabolic regulation. In intrinsic regulation, the metabolic pathway self-regulates to respond to changes in the levels of substrates or products; for example, a decrease in the amount of product can increase the flux through the pathway to compensate.[96] This type of regulation often involves allosteric regulation of the activities of multiple enzymes in the pathway.[98] Extrinsic control involves a cell in a multicellular organism changing its metabolism in response to signals from other cells. These signals are usually in the form of soluble messengers such as hormones and growth factors and are detected by specific receptors on the cell surface.[99] These signals are then transmitted inside the cell by second messenger systems that often involved the phosphorylation of proteins.[100]
A very well understood example of extrinsic control is the regulation of glucose metabolism by the hormone insulin.[101] Insulin is produced in response to rises in blood glucose levels. Binding of the hormone to insulin receptors on cells then activates a cascade of protein kinases that cause the cells to take up glucose and convert it into storage molecules such as fatty acids and glycogen.[102] The metabolism of glycogen is controlled by activity of phosphorylase, the enzyme that breaks down glycogen, and glycogen synthase, the enzyme that makes it. These enzymes are regulated in a reciprocal fashion, with phosphorylation inhibiting glycogen synthase, but activating phosphorylase. Insulin causes glycogen synthesis by activating protein phosphatases and producing a decrease in the phosphorylation of these enzymes.[103]
Evolution
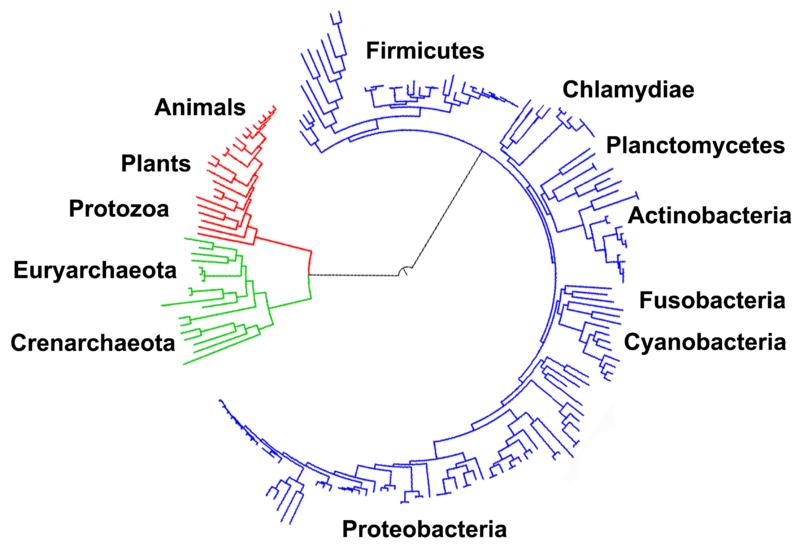
The central pathways of metabolism described above, such as glycolysis and the citric acid cycle, are present in all three domains of living things and were present in the last universal ancestor.[104][2] This universal ancestral cell was prokaryotic and probably a methanogen that had extensive amino acid, nucleotide, carbohydrate and lipid metabolism.[105][106] The retention of these ancient pathways during later evolution may be the result of these reactions being an optimal solution to their particular metabolic problems, with pathways such as glycolysis and the citric acid cycle producing their end products highly efficiently and in a minimal number of steps.[3][4] The first pathways of enzyme-based metabolism may have been parts of purine nucleotide metabolism, with previous metabolic pathways being part of the ancient RNA world.[107]
Many models have been proposed to describe the mechanisms by which novel metabolic pathways evolve. These include the sequential addition of novel enzymes to a short ancestral pathway, the duplication and then divergence of entire pathways as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway.[108] The relative importance of these mechanisms is unclear, but genomic studies have shown that enzymes in a pathway are likely to have a shared ancestry, suggesting that many pathways have evolved in a step-by-step fashion with novel functions being created from pre-existing steps in the pathway.[109] Another possibility is that some parts of metabolism might exist as "modules" that can be reused in different pathways and perform similar functions on different molecules.[110]
The evolution of organisms can also produce the loss of metabolic pathways. For example, in some parasites metabolic processes that are not essential for survival are lost and preformed amino acids, nucleotides and carbohydrates may instead be scavenged from the host.[111] Similar reduced metabolic capabilities are seen in endosymbiotic organisms.[112]
Investigation and manipulation
Classically, metabolism is studied by a reductionist approach that focuses on a single metabolic pathway. Particularly valuable is the use of radioactive tracers at the whole-organism, tissue and cellular levels, which define the paths from precursors to final products by identifying radioactively-labelled intermediates and products.[113] The enzymes that catalyze these chemical reactions can then be purified and their kinetics and responses to inhibitors investigated. A parallel approach is to identify the small molecules in a cell or tissue; the complete set of these molecules is called the metabolome. Overall, these studies give a good view of the structure and function of simple metabolic pathways, but are inadequate when applied to more complex systems such as the metabolism of a complete cell.[114]
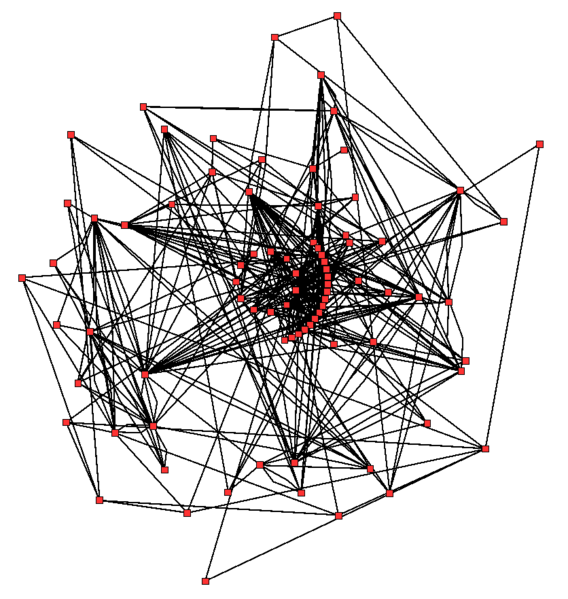
An idea of the complexity of the metabolic networks in cells that contain thousands of different enzymes is given by the figure showing the interactions between just 43 proteins and 40 metabolites to the right: the sequences of genomes provide lists containing anything up to 45,000 genes.[115] However, it is now possible to use this genomic data to reconstruct complete networks of biochemical reactions and produce more holistic mathematical models that may explain and predict their behavior.[116] These models are especially powerful when used to integrate the pathway and metabolite data obtained through classical methods with data on gene expression from proteomic and DNA microarray studies.[117]
A major technological application of this information is metabolic engineering. Here, organisms such as yeast, plants or bacteria are genetically-modified to make them more useful in biotechnology and aid the production of drugs such as antibiotics or industrial chemicals such as 1,3-propanediol and shikimic acid.[118] These genetic modifications usually aim to reduce the amount of energy used to produce the product, increase yields and reduce the production of wastes.[119]
History

The term metabolism is derived from the Greek Μεταβολισμός – "Metabolismos" for "change", or "overthrow".[120] The history of the scientific study of metabolism spans 400 years and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry. The first controlled experiments in human metabolism were published by Santorio Santorio in 1614 in his book Ars de statica medecina.[121] He described how he weighed himself before and after eating, sleeping, working, sex, fasting, drinking, and excreting. He found that most of the food he took in was lost through what he called "insensible perspiration".
In these early studies, the mechanisms of these metabolic processes had not been identified and a vital force was thought to animate living tissue.[122] In the 19th century, when studying the fermentation of sugar to alcohol by yeast, Louis Pasteur concluded that fermentation was catalyzed by substances within the yeast cells he called "ferments". He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells."[123] This discovery, along with the publication by Friedrich Wöhler in 1828 of the chemical synthesis of urea,[124] proved that the organic compounds and chemical reactions found in cells were no different in principle than any other part of chemistry.
It was the discovery of enzymes at the beginning of the 20th century by Eduard Buchner that separated the study of the chemical reactions of metabolism from the biological study of cells, and marked the beginnings of biochemistry.[125] The mass of biochemical knowledge grew rapidly throughout the early 20th century. One of the most prolific of these modern biochemists was Hans Krebs who made huge contributions to the study of metabolism.[126] He discovered the urea cycle and later, working with Hans Kornberg, the citric acid cycle and the glyoxylate cycle.[127][62] Modern biochemical research has been greatly aided by the development of new techniques such as chromatography, X-ray diffraction, NMR spectroscopy, radioisotopic labelling, electron microscopy and molecular dynamics simulations. These techniques have allowed the discovery and detailed analysis of the many molecules and metabolic pathways in cells.
See also
- Inborn error of metabolism
- Basal metabolic rate
- Thermic effect of food
- Iron-sulfur world theory, a "metabolism first" theory of the origin of life.
- Calorimetry
- Respirometry
- Anthropogenic metabolism
References
- ↑ Friedrich C (1998). "Physiology and genetics of sulfur-oxidizing bacteria". Adv Microb Physiol. 39: 235–89. PMID 9328649.
- ↑ 2.0 2.1 Smith E, Morowitz H (2004). "Universality in intermediary metabolism". Proc Natl Acad Sci U S A. 101 (36): 13168–73. PMID 15340153.
- ↑ 3.0 3.1 Ebenhöh O, Heinrich R (2001). "Evolutionary optimization of metabolic pathways. Theoretical reconstruction of the stoichiometry of ATP and NADH producing systems". Bull Math Biol. 63 (1): 21–55. PMID 11146883.
- ↑ 4.0 4.1 Meléndez-Hevia E, Waddell T, Cascante M (1996). "The puzzle of the Krebs citric acid cycle: assembling the pieces of chemically feasible reactions, and opportunism in the design of metabolic pathways during evolution". J Mol Evol. 43 (3): 293–303. PMID 8703096.
- ↑ Michie K, Löwe J (2006). "Dynamic filaments of the bacterial cytoskeleton". Annu Rev Biochem. 75: 467–92. PMID 16756499.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Nelson, David L. (2005). Lehninger Principles of Biochemistry. New York: W. H. Freeman and company. p. 841. ISBN 0-7167-4339-6. Unknown parameter
|coauthors=ignored (help) - ↑ Fahy E, Subramaniam S, Brown H, Glass C, Merrill A, Murphy R, Raetz C, Russell D, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze M, White S, Witztum J, Dennis E (2005). "A comprehensive classification system for lipids". J Lipid Res. 46 (5): 839–61. PMID 15722563.
- ↑ "Nomenclature of Lipids". IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Retrieved 2007-03-08.
- ↑ Hegardt F (1999). "Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis". Biochem J. 338 (Pt 3): 569–82. PMID 10051425.
- ↑ Raman R, Raguram S, Venkataraman G, Paulson J, Sasisekharan R (2005). "Glycomics: an integrated systems approach to structure-function relationships of glycans". Nat Methods. 2 (11): 817–24. PMID 16278650.
- ↑ Sierra S, Kupfer B, Kaiser R (2005). "Basics of the virology of HIV-1 and its replication". J Clin Virol. 34 (4): 233–44. PMID 16198625.
- ↑ 12.0 12.1 Wimmer M, Rose I (1978). "Mechanisms of enzyme-catalyzed group transfer reactions". Annu Rev Biochem. 47: 1031–78. PMID 354490.
- ↑ Mitchell P (1979). "The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems". Eur J Biochem. 95 (1): 1–20. PMID 378655.
- ↑ 14.0 14.1 14.2 14.3 Dimroth P, von Ballmoos C, Meier T (2006). "Catalytic and mechanical cycles in F-ATP synthases. Fourth in the Cycles Review Series". EMBO Rep. 7 (3): 276–82. PMID 16607397.
- ↑ Coulston, Ann; Kerner, John; Hattner, JoAnn; Srivastava, Ashini (2006), "Nutrition Principles and Clinical Nutrition", Stanford School of Medicine Nutrition Courses, SUMMIT
- ↑ Pollak N, Dölle C, Ziegler M (2007). "The power to reduce: pyridine nucleotides—small molecules with a multitude of functions". Biochem J. 402 (2): 205–18. PMID 17295611.
- ↑ 17.0 17.1 Heymsfield S, Waki M, Kehayias J, Lichtman S, Dilmanian F, Kamen Y, Wang J, Pierson R (1991). "Chemical and elemental analysis of humans in vivo using improved body composition models". Am J Physiol. 261 (2 Pt 1): E190–8. PMID 1872381.
- ↑ Sychrová H (2004). "Yeast as a model organism to study transport and homeostasis of alkali metal cations" (PDF). Physiol Res. 53 Suppl 1: S91–8. PMID 15119939.
- ↑ Levitan I (1988). "Modulation of ion channels in neurons and other cells". Annu Rev Neurosci. 11: 119–36. PMID 2452594.
- ↑ Dulhunty A (2006). "Excitation-contraction coupling from the 1950s into the new millennium". Clin Exp Pharmacol Physiol. 33 (9): 763–72. PMID 16922804.
- ↑ Mahan D, Shields R (1998). "Macro- and micromineral composition of pigs from birth to 145 kilograms of body weight". J Anim Sci. 76 (2): 506–12. PMID 9498359.
- ↑ Husted S, Mikkelsen B, Jensen J, Nielsen N (2004). "Elemental fingerprint analysis of barley (Hordeum vulgare) using inductively coupled plasma mass spectrometry, isotope-ratio mass spectrometry, and multivariate statistics". Anal Bioanal Chem. 378 (1): 171–82. PMID 14551660.
- ↑ Finney L, O'Halloran T (2003). "Transition metal speciation in the cell: insights from the chemistry of metal ion receptors". Science. 300 (5621): 931–6. PMID 12738850.
- ↑ Cousins R, Liuzzi J, Lichten L (2006). "Mammalian zinc transport, trafficking, and signals". J Biol Chem. 281 (34): 24085–9. PMID 16793761.
- ↑ Dunn L, Rahmanto Y, Richardson D (2007). "Iron uptake and metabolism in the new millennium". Trends Cell Biol. 17 (2): 93–100. PMID 17194590.
- ↑ Nealson K, Conrad P (1999). "Life: past, present and future". Philos Trans R Soc Lond B Biol Sci. 354 (1392): 1923–39. PMID 10670014.
- ↑ Häse C, Finkelstein R (1993). "Bacterial extracellular zinc-containing metalloproteases". Microbiol Rev. 57 (4): 823–37. PMID 8302217.
- ↑ Gupta R, Gupta N, Rathi P (2004). "Bacterial lipases: an overview of production, purification and biochemical properties". Appl Microbiol Biotechnol. 64 (6): 763–81. PMID 14966663.
- ↑ Hoyle T (1997). "The digestive system: linking theory and practice". Br J Nurs. 6 (22): 1285–91. PMID 9470654.
- ↑ Souba W, Pacitti A (1992). "How amino acids get into cells: mechanisms, models, menus, and mediators". JPEN J Parenter Enteral Nutr. 16 (6): 569–78. PMID 1494216.
- ↑ Barrett M, Walmsley A, Gould G (1999). "Structure and function of facilitative sugar transporters". Curr Opin Cell Biol. 11 (4): 496–502. PMID 10449337.
- ↑ Bell G, Burant C, Takeda J, Gould G (1993). "Structure and function of mammalian facilitative sugar transporters". J Biol Chem. 268 (26): 19161–4. PMID 8366068.
- ↑ 33.0 33.1 Bouché C, Serdy S, Kahn C, Goldfine A (2004). "The cellular fate of glucose and its relevance in type 2 diabetes". Endocr Rev. 25 (5): 807–30. PMID 15466941.
- ↑ Sakami W, Harrington H (1963). "Amino acid metabolism". Annu Rev Biochem. 32: 355–98. PMID 14144484.
- ↑ Brosnan J (2000). "Glutamate, at the interface between amino acid and carbohydrate metabolism". J Nutr. 130 (4S Suppl): 988S–90S. PMID 10736367.
- ↑ Young V, Ajami A (2001). "Glutamine: the emperor or his clothes?". J Nutr. 131 (9 Suppl): 2449S–59S, discussion 2486S-7S. PMID 11533293.
- ↑ Hosler J, Ferguson-Miller S, Mills D (2006). "Energy transduction: proton transfer through the respiratory complexes". Annu Rev Biochem. 75: 165–87. PMID 16756489.
- ↑ Schultz B, Chan S (2001). "Structures and proton-pumping strategies of mitochondrial respiratory enzymes". Annu Rev Biophys Biomol Struct. 30: 23–65. PMID 11340051.
- ↑ Capaldi R, Aggeler R (2002). "Mechanism of the F(1)F(0)-type ATP synthase, a biological rotary motor". Trends Biochem Sci. 27 (3): 154–60. PMID 11893513.
- ↑ Friedrich B, Schwartz E (1993). "Molecular biology of hydrogen utilization in aerobic chemolithotrophs". Annu Rev Microbiol. 47: 351–83. PMID 8257102.
- ↑ Friedrich C (1998). "Physiology and genetics of sulfur-oxidizing bacteria". Adv Microb Physiol. 39: 235–89. PMID 9328649.
- ↑ Weber K, Achenbach L, Coates J (2006). "Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction". Nat Rev Microbiol. 4 (10): 752–64. PMID 16980937.
- ↑ Jetten M, Strous M, van de Pas-Schoonen K, Schalk J, van Dongen U, van de Graaf A, Logemann S, Muyzer G, van Loosdrecht M, Kuenen J (1998). "The anaerobic oxidation of ammonium". FEMS Microbiol Rev. 22 (5): 421–37. PMID 9990725.
- ↑ Simon J (2002). "Enzymology and bioenergetics of respiratory nitrite ammonification". FEMS Microbiol Rev. 26 (3): 285–309. PMID 12165429.
- ↑ Conrad R (1996). "Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO)". Microbiol Rev. 60 (4): 609–40. PMID 8987358.
- ↑ Barea J, Pozo M, Azcón R, Azcón-Aguilar C (2005). "Microbial co-operation in the rhizosphere". J Exp Bot. 56 (417): 1761–78. PMID 15911555.
- ↑ van der Meer M, Schouten S, Bateson M, Nübel U, Wieland A, Kühl M, de Leeuw J, Sinninghe Damsté J, Ward D (2005). "Diel variations in carbon metabolism by green nonsulfur-like bacteria in alkaline siliceous hot spring microbial mats from Yellowstone National Park". Appl Environ Microbiol. 71 (7): 3978–86. PMID 16000812.
- ↑ Tichi M, Tabita F (2001). "Interactive control of Rhodobacter capsulatus redox-balancing systems during phototrophic metabolism". J Bacteriol. 183 (21): 6344–54. PMID 11591679.
- ↑ Allen J, Williams J (1998). "Photosynthetic reaction centers". FEBS Lett. 438 (1–2): 5–9. PMID 9821949.
- ↑ Nelson N, Ben-Shem A (2004). "The complex architecture of oxygenic photosynthesis". Nat Rev Mol Cell Biol. 5 (12): 971–82. PMID 15573135.
- ↑ Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004). "Cyclic electron flow around photosystem I is essential for photosynthesis". Nature. 429 (6991): 579–82. PMID 15175756.
- ↑ Miziorko H, Lorimer G (1983). "Ribulose-1,5-bisphosphate carboxylase-oxygenase". Annu Rev Biochem. 52: 507–35. PMID 6351728.
- ↑ Dodd A, Borland A, Haslam R, Griffiths H, Maxwell K (2002). "Crassulacean acid metabolism: plastic, fantastic". J Exp Bot. 53 (369): 569–80. PMID 11886877.
- ↑ Hügler M, Wirsen C, Fuchs G, Taylor C, Sievert S (2005). "Evidence for autotrophic CO2 fixation via the reductive tricarboxylic acid cycle by members of the epsilon subdivision of proteobacteria". J Bacteriol. 187 (9): 3020–7. PMID 15838028.
- ↑ Strauss G, Fuchs G (1993). "Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle". Eur J Biochem. 215 (3): 633–43. PMID 8354269.
- ↑ Wood H (1991). "Life with CO or CO2 and H2 as a source of carbon and energy". FASEB J. 5 (2): 156–63. PMID 1900793.
- ↑ Shively J, van Keulen G, Meijer W (1998). "Something from almost nothing: carbon dioxide fixation in chemoautotrophs". Annu Rev Microbiol. 52: 191–230. PMID 9891798.
- ↑ Boiteux A, Hess B (1981). "Design of glycolysis". Philos Trans R Soc Lond B Biol Sci. 293 (1063): 5–22. PMID 6115423.
- ↑ Pilkis S, el-Maghrabi M, Claus T (1990). "Fructose-2,6-bisphosphate in control of hepatic gluconeogenesis. From metabolites to molecular genetics". Diabetes Care. 13 (6): 582–99. PMID 2162755.
- ↑ 60.0 60.1 Ensign S (2006). "Revisiting the glyoxylate cycle: alternate pathways for microbial acetate assimilation". Mol Microbiol. 61 (2): 274–6. PMID 16856935.
- ↑ Finn P, Dice J (2006). "Proteolytic and lipolytic responses to starvation". Nutrition. 22 (7–8): 830–44. PMID 16815497.
- ↑ 62.0 62.1 Kornberg H, Krebs H (1957). "Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle". Nature. 179 (4568): 988–91. PMID 13430766.
- ↑ Rademacher T, Parekh R, Dwek R (1988). "Glycobiology". Annu Rev Biochem. 57: 785–838. PMID 3052290.
- ↑ Opdenakker G, Rudd P, Ponting C, Dwek R (1993). "Concepts and principles of glycobiology". FASEB J. 7 (14): 1330–7. PMID 8224606.
- ↑ McConville M, Menon A (2000). "Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids (review)". Mol Membr Biol. 17 (1): 1–16. PMID 10824734.
- ↑ Chirala S, Wakil S (2004). "Structure and function of animal fatty acid synthase". Lipids. 39 (11): 1045–53. PMID 15726818.
- ↑ White S, Zheng J, Zhang Y (2005). "The structural biology of type II fatty acid biosynthesis". Annu Rev Biochem. 74: 791–831. PMID 15952903.
- ↑ Ohlrogge J, Jaworski J (1997). "Regulation of fatty acid synthesis". Annu Rev Plant Physiol Plant Mol Biol. 48: 109–136. PMID 15012259.
- ↑ Dubey V, Bhalla R, Luthra R (2003). "An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants" (PDF). J Biosci. 28 (5): 637–46. PMID 14517367.
- ↑ 70.0 70.1 Kuzuyama T, Seto H (2003). "Diversity of the biosynthesis of the isoprene units". Nat Prod Rep. 20 (2): 171–83. PMID 12735695.
- ↑ Grochowski L, Xu H, White R (2006). "Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate". J Bacteriol. 188 (9): 3192–8. PMID 16621811.
- ↑ Lichtenthaler H (1999). "The 1-Ddeoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants". Annu Rev Plant Physiol Plant Mol Biol. 50: 47–65. PMID 15012203.
- ↑ 73.0 73.1 Schroepfer G (1981). "Sterol biosynthesis". Annu Rev Biochem. 50: 585–621. PMID 7023367.
- ↑ Lees N, Skaggs B, Kirsch D, Bard M (1995). "Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae—a review". Lipids. 30 (3): 221–6. PMID 7791529.
- ↑ Guyton, Arthur C. (2006). Textbook of Medical Physiology. Philadelphia: Elsevier. pp. 855–6. ISBN 0-7216-0240-1. Unknown parameter
|coauthors=ignored (help) - ↑ Ibba M, Söll D (2001). "The renaissance of aminoacyl-tRNA synthesis". EMBO Rep. 2 (5): 382–7. PMID 11375928.
- ↑ Lengyel P, Söll D (1969). "Mechanism of protein biosynthesis". Bacteriol Rev. 33 (2): 264–301. PMID 4896351.
- ↑ 78.0 78.1 Rudolph F (1994). "The biochemistry and physiology of nucleotides". J Nutr. 124 (1 Suppl): 124S–127S. PMID 8283301. Zrenner R, Stitt M, Sonnewald U, Boldt R (2006). "Pyrimidine and purine biosynthesis and degradation in plants". Annu Rev Plant Biol. 57: 805–36. PMID 16669783.
- ↑ Stasolla C, Katahira R, Thorpe T, Ashihara H (2003). "Purine and pyrimidine nucleotide metabolism in higher plants". J Plant Physiol. 160 (11): 1271–95. PMID 14658380.
- ↑ Smith J (1995). "Enzymes of nucleotide synthesis". Curr Opin Struct Biol. 5 (6): 752–7. PMID 8749362.
- ↑ Testa B, Krämer S (2006). "The biochemistry of drug metabolism—an introduction: part 1. Principles and overview". Chem Biodivers. 3 (10): 1053–101. PMID 17193224.
- ↑ Danielson P (2002). "The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans". Curr Drug Metab. 3 (6): 561–97. PMID 12369887.
- ↑ King C, Rios G, Green M, Tephly T (2000). "UDP-glucuronosyltransferases". Curr Drug Metab. 1 (2): 143–61. PMID 11465080.
- ↑ Sheehan D, Meade G, Foley V, Dowd C (2001). "Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily". Biochem J. 360 (Pt 1): 1–16. PMID 11695986.
- ↑ Galvão T, Mohn W, de Lorenzo V (2005). "Exploring the microbial biodegradation and biotransformation gene pool". Trends Biotechnol. 23 (10): 497–506. PMID 16125262.
- ↑ Janssen D, Dinkla I, Poelarends G, Terpstra P (2005). "Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities". Environ Microbiol. 7 (12): 1868–82. PMID 16309386.
- ↑ Davies K (1995). "Oxidative stress: the paradox of aerobic life". Biochem Soc Symp. 61: 1–31. PMID 8660387.
- ↑ Tu B, Weissman J (2004). "Oxidative protein folding in eukaryotes: mechanisms and consequences". J Cell Biol. 164 (3): 341–6. PMID 14757749.
- ↑ Sies H (1997). "Oxidative stress: oxidants and antioxidants" (PDF). Exp Physiol. 82 (2): 291–5. PMID 9129943.
- ↑ Vertuani S, Angusti A, Manfredini S (2004). "The antioxidants and pro-antioxidants network: an overview". Curr Pharm Des. 10 (14): 1677–94. PMID 15134565.
- ↑ von Stockar U, Liu J (1999). "Does microbial life always feed on negative entropy? Thermodynamic analysis of microbial growth". Biochim Biophys Acta. 1412 (3): 191–211. PMID 10482783.
- ↑ Demirel Y, Sandler S (2002). "Thermodynamics and bioenergetics". Biophys Chem. 97 (2–3): 87–111. PMID 12050002.
- ↑ Albert R (2005). "Scale-free networks in cell biology". J Cell Sci. 118 (Pt 21): 4947–57. PMID 16254242.
- ↑ Brand M (1997). "Regulation analysis of energy metabolism". J Exp Biol. 200 (Pt 2): 193–202. PMID 9050227.
- ↑ Soyer O, Salathé M, Bonhoeffer S (2006). "Signal transduction networks: topology, response and biochemical processes". J Theor Biol. 238 (2): 416–25. PMID 16045939.
- ↑ 96.0 96.1 Salter M, Knowles R, Pogson C (1994). "Metabolic control". Essays Biochem. 28: 1–12. PMID 7925313.
- ↑ Westerhoff H, Groen A, Wanders R (1984). "Modern theories of metabolic control and their applications (review)". Biosci Rep. 4 (1): 1–22. PMID 6365197.
- ↑ Fell D, Thomas S (1995). "Physiological control of metabolic flux: the requirement for multisite modulation". Biochem J. 311 (Pt 1): 35–9. PMID 7575476.
- ↑ Hendrickson W (2005). "Transduction of biochemical signals across cell membranes". Q Rev Biophys. 38 (4): 321–30. PMID 16600054.
- ↑ Cohen P (2000). "The regulation of protein function by multisite phosphorylation—a 25 year update". Trends Biochem Sci. 25 (12): 596–601. PMID 11116185.
- ↑ Lienhard G, Slot J, James D, Mueckler M (1992). "How cells absorb glucose". Sci Am. 266 (1): 86–91. PMID 1734513.
- ↑ Roach P (2002). "Glycogen and its metabolism". Curr Mol Med. 2 (2): 101–20. PMID 11949930.
- ↑ Newgard C, Brady M, O'Doherty R, Saltiel A (2000). "Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1" (PDF). Diabetes. 49 (12): 1967–77. PMID 11117996.
- ↑ Romano A, Conway T (1996). "Evolution of carbohydrate metabolic pathways". Res Microbiol. 147 (6–7): 448–55. PMID 9084754.
- ↑ Koch A (1998). "How did bacteria come to be?". Adv Microb Physiol. 40: 353–99. PMID 9889982.
- ↑ Ouzounis C, Kyrpides N (1996). "The emergence of major cellular processes in evolution". FEBS Lett. 390 (2): 119–23. PMID 8706840.
- ↑ Caetano-Anolles G, Kim HS, Mittenthal JE (2007). "The origin of modern metabolic networks inferred from phylogenomic analysis of protein architecture". Proc Natl Acad Sci USA. 104 (22): 9358–63. PMID 17517598.
- ↑ Schmidt S, Sunyaev S, Bork P, Dandekar T (2003). "Metabolites: a helping hand for pathway evolution?". Trends Biochem Sci. 28 (6): 336–41. PMID 12826406.
- ↑ Light S, Kraulis P (2004). "Network analysis of metabolic enzyme evolution in Escherichia coli". BMC Bioinformatics. 5: 15. PMID 15113413. Alves R, Chaleil R, Sternberg M (2002). "Evolution of enzymes in metabolism: a network perspective". J Mol Biol. 320 (4): 751–70. PMID 12095253.
- ↑ Spirin V, Gelfand M, Mironov A, Mirny L (2006). "A metabolic network in the evolutionary context: multiscale structure and modularity". Proc Natl Acad Sci U S A. 103 (23): 8774–9. PMID 16731630.
- ↑ Lawrence J (2005). "Common themes in the genome strategies of pathogens". Curr Opin Genet Dev. 15 (6): 584–8. PMID 16188434. Wernegreen J (2005). "For better or worse: genomic consequences of intracellular mutualism and parasitism". Curr Opin Genet Dev. 15 (6): 572–83. PMID 16230003.
- ↑ Pál C, Papp B, Lercher M, Csermely P, Oliver S, Hurst L (2006). "Chance and necessity in the evolution of minimal metabolic networks". Nature. 440 (7084): 667–70. PMID 16572170.
- ↑ Rennie M (1999). "An introduction to the use of tracers in nutrition and metabolism". Proc Nutr Soc. 58 (4): 935–44. PMID 10817161.
- ↑ Phair R (1997). "Development of kinetic models in the nonlinear world of molecular cell biology". Metabolism. 46 (12): 1489–95. PMID 9439549.
- ↑ Sterck L, Rombauts S, Vandepoele K, Rouzé P, Van de Peer Y (2007). "How many genes are there in plants (... and why are they there)?". Curr Opin Plant Biol. 10 (2): 199–203. PMID 17289424.
- ↑ Borodina I, Nielsen J (2005). "From genomes to in silico cells via metabolic networks". Curr Opin Biotechnol. 16 (3): 350–5. PMID 15961036.
- ↑ Gianchandani E, Brautigan D, Papin J (2006). "Systems analyses characterize integrated functions of biochemical networks". Trends Biochem Sci. 31 (5): 284–91. PMID 16616498.
- ↑ Thykaer J, Nielsen J (2003). "Metabolic engineering of beta-lactam production". Metab Eng. 5 (1): 56–69. PMID 12749845. González-Pajuelo M, Meynial-Salles I, Mendes F, Andrade J, Vasconcelos I, Soucaille P (2005). "Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol". Metab Eng. 7 (5–6): 329–36. PMID 16095939. Krämer M, Bongaerts J, Bovenberg R, Kremer S, Müller U, Orf S, Wubbolts M, Raeven L (2003). "Metabolic engineering for microbial production of shikimic acid". Metab Eng. 5 (4): 277–83. PMID 14642355.
- ↑ Koffas M, Roberge C, Lee K, Stephanopoulos G (1999). "Metabolic engineering". Annu Rev Biomed Eng. 1: 535–57. PMID 11701499.
- ↑ "Metabolism". The Online Etymology Dictionary. Retrieved 2007-02-20.
- ↑ Eknoyan G (1999). "Santorio Sanctorius (1561–1636) - founding father of metabolic balance studies". Am J Nephrol. 19 (2): 226–33. PMID 10213823.
- ↑ Williams, H. S. (1904) A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences Harper and Brothers (New York) Retrieved on 2007-03-26
- ↑ Dubos J. (1951). "Louis Pasteur: Free Lance of Science, Gollancz. Quoted in Manchester K. L. (1995) Louis Pasteur (1822 – 1895)—chance and the prepared mind". Trends Biotechnol. 13 (12): 511–515. PMID 8595136.
- ↑ Kinne-Saffran E, Kinne R (1999). "Vitalism and synthesis of urea. From Friedrich Wöhler to Hans A. Krebs". Am J Nephrol. 19 (2): 290–4. PMID 10213830.
- ↑ Eduard Buchner's 1907 Nobel lecture at http://nobelprize.org Accessed 2007-03-20
- ↑ Kornberg H (2000). "Krebs and his trinity of cycles". Nat Rev Mol Cell Biol. 1 (3): 225–8. PMID 11252898.
- ↑ Krebs H A, Henseleit K (1932) "Untersuchungen über die Harnstoffbildung im tierkorper." Z. Physiol. Chem. 210, 33 – 66. Krebs H, Johnson W (1937). "Metabolism of ketonic acids in animal tissues". Biochem J. 31 (4): 645–60. PMID 16746382.
Further reading
Introductory
- Rose, S. and Mileusnic, R., The Chemistry of Life. (Penguin Press Science, 1999), ISBN 0-14027-273-9
- Schneider, E. D. and Sagan, D., Into the Cool: Energy Flow, Thermodynamics, and Life. (University Of Chicago Press, 2005), ISBN 0-22673-936-8
- Lane, N., Oxygen: The Molecule that Made the World. (Oxford University Press, USA, 2004), ISBN 0-19860-783-0
Advanced
- Price, N. and Stevens, L., Fundamentals of Enzymology: Cell and Molecular Biology of Catalytic Proteins. (Oxford University Press, 1999), ISBN 0-19850-229-X
- Berg, J. Tymoczko, J. and Stryer, L., Biochemistry. (W. H. Freeman and Company, 2002), ISBN 0-71674-955-6
- Cox, M. and Nelson, D. L., Lehninger Principles of Biochemistry. (Palgrave Macmillan, 2004), ISBN 0-71674-339-6
- Brock, T. D. Madigan, M. T. Martinko, J. and Parker J., Brock's Biology of Microorganisms. (Benjamin Cummings, 2002), ISBN 0-13066-271-2
- Da Silva, J.J.R.F. and Williams, R. J. P., The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. (Clarendon Press, 1991), ISBN 0-19855-598-9
- Nicholls, D. G. and Ferguson, S. J., Bioenergetics. (Academic Press Inc., 2002), ISBN 0-12518-121-3
External links
- Interactive Flow Chart of the Major Metabolic Pathways
- Metabolism, Cellular Respiration and Photosynthesis The Virtual Library of Biochemistry and Cell Biology at biochemweb.org
- The Biochemistry of Metabolism
- Advanced Animal Metabolism Calculators/ Interactive Learning Tools
- Microbial metabolism Simple overview. School level.
- Metabolic Pathways of Biochemistry Graphical representations of major metabolic pathways.
- Chemistry for biologists Introduction to the chemistry of metabolism. School level.
- Sparknotes SAT biochemistry Overview of biochemistry. School level.
- MIT Biology Hypertextbook Undergraduate-level guide to molecular biology.
- Article on metabolism at The Encyclopœdia Britannica Concentrates on human metabolism (Free access).
- Topics in Medical Biochemistry Guide to human metabolic pathways. School level.
- THE Medical Biochemistry Page Comprehensive resource on human metabolism.
- The BioCyc Collection of Pathway/Genome Databases
- Flow Chart of Metabolic Pathways at ExPASy
- The KEGG PATHWAY Database
- IUBMB-Nicholson Metabolic Pathways Chart
- Reactome - a knowledgebase of biological processes
- Guide to Glycolysis School level.
- Template:Wayback
- Downloadable guide to photosynthesis School level.
- What is Photosynthesis? Collection of photosynthesis articles and resources.
Template:Link FA Template:Link FA
ar:استقلاب ca:Metabolisme cs:Metabolismus da:Stofskifte de:Stoffwechsel et:Metabolism eo:Metabolo fa:دگرگشت gl:Metabolismo ko:신진대사 io:Metabolio id:Metabolisme is:Efnaskipti it:Metabolismo he:מטבוליזם lb:Metabolismus lt:metabolizmas mk:Метаболизам nl:Stofwisseling no:Stoffskifte nn:Stoffskifte sq:Metabolizmi simple:Metabolism sk:Látková premena su:Métabolisme fi:Aineenvaihdunta sv:Metabolism th:กระบวนการสร้างและสลาย uk:Обмін речовин