Malate
|
WikiDoc Resources for Malate |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Malate at Clinical Trials.gov Clinical Trials on Malate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Malate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Malate Risk calculators and risk factors for Malate
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
- For the district in Manila, see Malate, Manila.
Overview
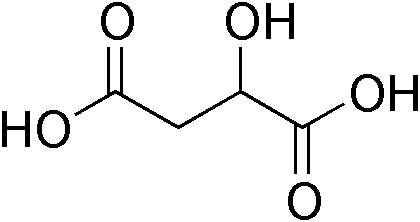
Malate (O−OC-CH2-CH(OH)-COO−) is the ionized form of malic acid. It is an important chemical compound in biochemistry. In the C4 carbon fixation process, malate is a source of CO2 in the Calvin cycle.
In the citric acid cycle, (S)-malate is an intermediate formed by the addition of an -OH group on the si face of fumarate; it can also be formed from pyruvate via anaplerotic reactions. Malate dehydrogenase catalyzes the reversible conversion of malate into oxaloacetate using NAD as a cofactor.
Malate is also produced from starch in guard cells of plant leaves. A build up of malate leads to a low water potential. Water then flows into the guard cells causing the stoma to open. However, this process does not always induce the opening of stomas.