MALT lymphoma pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
In other sites, chronic immune stimulation is also suspected in the pathogenesis (e.g. association between chronic [[autoimmune diseases]] such as [[Sjögren's syndrome]] and [[Hashimoto's thyroiditis]], and MALT lymphoma of the [[salivary gland]] and the [[thyroid]]). | In other sites, chronic immune stimulation is also suspected in the pathogenesis (e.g. association between chronic [[autoimmune diseases]] such as [[Sjögren's syndrome]] and [[Hashimoto's thyroiditis]], and MALT lymphoma of the [[salivary gland]] and the [[thyroid]]). | ||
===Gross Pathology=== | |||
{{Infobox_Disease | | |||
Image = Gastric MALT lymphoma 2.jpg | | |||
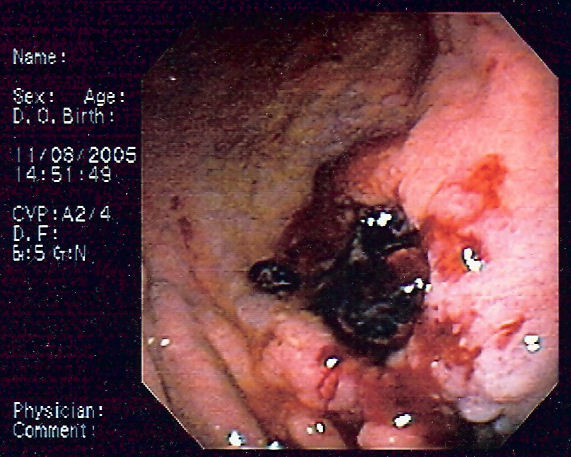
Caption = Endoscopic image of gastric MALT lymphoma taken in body of [[stomach]] in patient who presented with [[upper gastrointestinal bleed|upper GI hemorrhage]]. Appearance is similar to [[gastric ulcer]] with adherent clot. | | |||
}} | |||
===Microscopic pathology=== | ===Microscopic pathology=== | ||
Histologically, there is expansion of the marginal zone compartment with development of sheets of neoplastic small lymphoid cells.<ref>{{cite journal|last1=Taal|first1=B G|last2=Boot|first2=H|last3=van Heerde|first3=P|last4=de Jong|first4=D|last5=Hart|first5=A A|last6=Burgers|first6=J M|title=Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept.|journal=Gut|date=1 October 1996|volume=39|issue=4|pages=556–561|doi=10.1136/gut.39.4.556}}</ref> The morphology of the neoplastic cells is variable with small mature lymphocytes, cells resembling centrocytes (centrocyte like cells), or marginal zone/monocytoid B cells. Plasmacytoid or plasmacytic differentiation is frequent. Lymphoid follicles are ubiquitous to ''MALT lymphoma'' but may be indistinct as they are often overrun or colonized by the neoplastic cells. Large transformed B cells are present scattered among the small cell population. If these large cells are present in clusters or sheets, a diagnosis of associated large B-cell lymphoma should be considered. A characteristic feature of MALT lymphoma is the presence of neoplastic cells within epithelial structures with associated destruction of the glandular architecture to form lymphoepithelial lesions.<ref>{{cite book|last1=Janusz|first1=edited by Jankowski,|title=Handbook of Gastrointestinal Cancer|date=2012|publisher=John Wiley and Sons Ltd|location=Chicester|isbn=978-0-470-65624-2|pages=243–244|edition=2}}</ref> | Histologically, there is expansion of the marginal zone compartment with development of sheets of neoplastic small lymphoid cells.<ref>{{cite journal|last1=Taal|first1=B G|last2=Boot|first2=H|last3=van Heerde|first3=P|last4=de Jong|first4=D|last5=Hart|first5=A A|last6=Burgers|first6=J M|title=Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept.|journal=Gut|date=1 October 1996|volume=39|issue=4|pages=556–561|doi=10.1136/gut.39.4.556}}</ref> The morphology of the neoplastic cells is variable with small mature lymphocytes, cells resembling centrocytes (centrocyte like cells), or marginal zone/monocytoid B cells. Plasmacytoid or plasmacytic differentiation is frequent. Lymphoid follicles are ubiquitous to ''MALT lymphoma'' but may be indistinct as they are often overrun or colonized by the neoplastic cells. Large transformed B cells are present scattered among the small cell population. If these large cells are present in clusters or sheets, a diagnosis of associated large B-cell lymphoma should be considered. A characteristic feature of MALT lymphoma is the presence of neoplastic cells within epithelial structures with associated destruction of the glandular architecture to form lymphoepithelial lesions.<ref>{{cite book|last1=Janusz|first1=edited by Jankowski,|title=Handbook of Gastrointestinal Cancer|date=2012|publisher=John Wiley and Sons Ltd|location=Chicester|isbn=978-0-470-65624-2|pages=243–244|edition=2}}</ref> | ||
Revision as of 15:40, 3 September 2015
|
MALT lymphoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
MALT lymphoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of MALT lymphoma pathophysiology |
|
Risk calculators and risk factors for MALT lymphoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
MALT lymphoma (MALToma) is a form of lymphoma involving the mucosa-associated lymphoid tissue (MALT), frequently of the stomach, but virtually any mucosal site can be afflicted. It is a cancer originating from B cells in the marginal zone of the MALT
Pathophysiology
MALT lymphoma (MALToma) is a form of lymphoma involving the mucosa-associated lymphoid tissue (MALT), frequently of the stomach, but virtually any mucosal site can be afflicted. It is a cancer originating from B cells in the marginal zone of the MALT
Genetics
A t(11;18)(q21;q21) chromosomal translocation, giving rise to a AP12-MLT fusion gene, is predictive of poor response to eradication therapy. [1]
Two other genetic alterations, t(1;14)(p22;q32) and t(14;18)(q32;q21), which deregulate BCL10 and MALT1-genes respectively, and seem to turn-on the same pathway as API2-MLT (i.e., that of NF-kB).
Associations
Gastric MALT lymphoma is frequently associated (72-98%) with chronic inflammation as a result of the presence of Helicobacter pylori. [2]
In other sites, chronic immune stimulation is also suspected in the pathogenesis (e.g. association between chronic autoimmune diseases such as Sjögren's syndrome and Hashimoto's thyroiditis, and MALT lymphoma of the salivary gland and the thyroid).
Gross Pathology
| MALT lymphoma pathophysiology | |
 | |
|---|---|
| Endoscopic image of gastric MALT lymphoma taken in body of stomach in patient who presented with upper GI hemorrhage. Appearance is similar to gastric ulcer with adherent clot. |
Microscopic pathology
Histologically, there is expansion of the marginal zone compartment with development of sheets of neoplastic small lymphoid cells.[3] The morphology of the neoplastic cells is variable with small mature lymphocytes, cells resembling centrocytes (centrocyte like cells), or marginal zone/monocytoid B cells. Plasmacytoid or plasmacytic differentiation is frequent. Lymphoid follicles are ubiquitous to MALT lymphoma but may be indistinct as they are often overrun or colonized by the neoplastic cells. Large transformed B cells are present scattered among the small cell population. If these large cells are present in clusters or sheets, a diagnosis of associated large B-cell lymphoma should be considered. A characteristic feature of MALT lymphoma is the presence of neoplastic cells within epithelial structures with associated destruction of the glandular architecture to form lymphoepithelial lesions.[4]
References
- ↑ Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, Hamoudi R, Diss T, Dogan A, Megraud F, Rambaud J, Du M, Isaacson P (2001). "Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy". Lancet. 357 (9249): 39–40. PMID 11197361.
- ↑ Parsonnet J, Hansen S, Rodriguez L, Gelb A, Warnke R, Jellum E, Orentreich N, Vogelman J, Friedman G (1994). "Helicobacter pylori infection and gastric lymphoma". N Engl J Med. 330 (18): 1267–71. PMID 8145781.
- ↑ Taal, B G; Boot, H; van Heerde, P; de Jong, D; Hart, A A; Burgers, J M (1 October 1996). "Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept". Gut. 39 (4): 556–561. doi:10.1136/gut.39.4.556.
- ↑ Janusz, edited by Jankowski, (2012). Handbook of Gastrointestinal Cancer (2 ed.). Chicester: John Wiley and Sons Ltd. pp. 243–244. ISBN 978-0-470-65624-2.