Loracarbef: Difference between revisions
Gerald Chi (talk | contribs) |
Gerald Chi (talk | contribs) m (→Overview) |
||
| Line 53: | Line 53: | ||
==Overview== | ==Overview== | ||
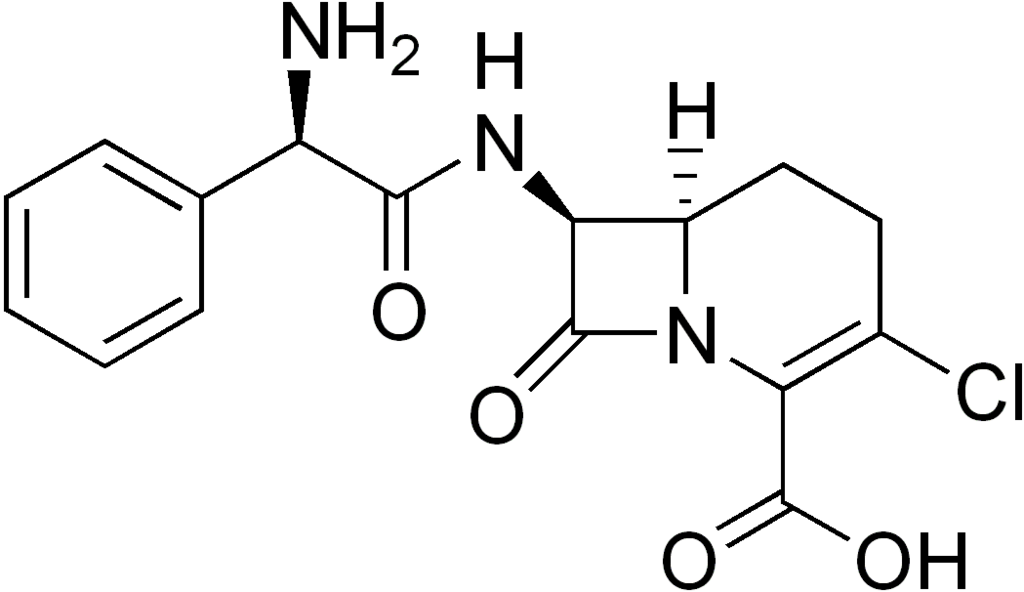
Loracarbef is | Loracarbef is a synthetic [[carbacephem]] antibiotic.<ref name="pmid8150976">{{cite journal |author=Biedenbach DJ, Jones RN |title=Predictive accuracy of disk diffusion test for Proteus vulgaris and Providencia species against five newer orally administered cephalosporins, cefdinir, cefetamet, cefprozil, cefuroxime, and loracarbef |journal=J. Clin. Microbiol. |volume=32 |issue=2|pages=559–62 |year=1994 |month=February |pmid=8150976 |pmc=263078 |doi= |url=http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=8150976}}</ref> Loracarbef has a spectrum of activity similar to that of the second generation [[cephalosporin]]s. It is an analogue of [[cefaclor]] from which the [[sulfur]] atom is replaced with a [[methylene]] group. Therefore loracarbef has a greater stability in solution and may be stored at room temperature. | ||
[[Diarrhea]] is the most common adverse effect. Side effects are more frequently seen with children under the age of twelve. It received FDA approval in 1991. Its use was discontinued in 2006. | |||
==Category== | ==Category== | ||
Revision as of 03:27, 31 December 2013
 | |
| Clinical data | |
|---|---|
| Trade names | Lorabid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 25% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H16ClN3O4 |
| Molar mass | 349.769 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Loracarbef is a synthetic carbacephem antibiotic.[1] Loracarbef has a spectrum of activity similar to that of the second generation cephalosporins. It is an analogue of cefaclor from which the sulfur atom is replaced with a methylene group. Therefore loracarbef has a greater stability in solution and may be stored at room temperature.
Diarrhea is the most common adverse effect. Side effects are more frequently seen with children under the age of twelve. It received FDA approval in 1991. Its use was discontinued in 2006.
Category
Carbacephem
US Brand Names
LORABID®
Mechanism of Action
Loracarbef is an oral, synthetic beta-lactam antibiotic of the carbacephem class. Loracarbef, like all beta-lactams and cephalosporins, inhibits penicillin binding proteins, enzymes that create the cross-linkage of the peptidoglycan polymer. This binding leads to interference with the formation and remodeling of the cell wall structure.
References
- ↑ Biedenbach DJ, Jones RN (1994). "Predictive accuracy of disk diffusion test for Proteus vulgaris and Providencia species against five newer orally administered cephalosporins, cefdinir, cefetamet, cefprozil, cefuroxime, and loracarbef". J. Clin. Microbiol. 32 (2): 559–62. PMC 263078. PMID 8150976. Unknown parameter
|month=ignored (help)
External links
- Pages with script errors
- Pages with citations using unsupported parameters
- Template:drugs.com link with non-standard subpage
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Antibiotics
- Wikinfect