Loracarbef: Difference between revisions
Jump to navigation
Jump to search
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | |||
| Verifiedfields = changed | |||
| verifiedrevid = 462093698 | |||
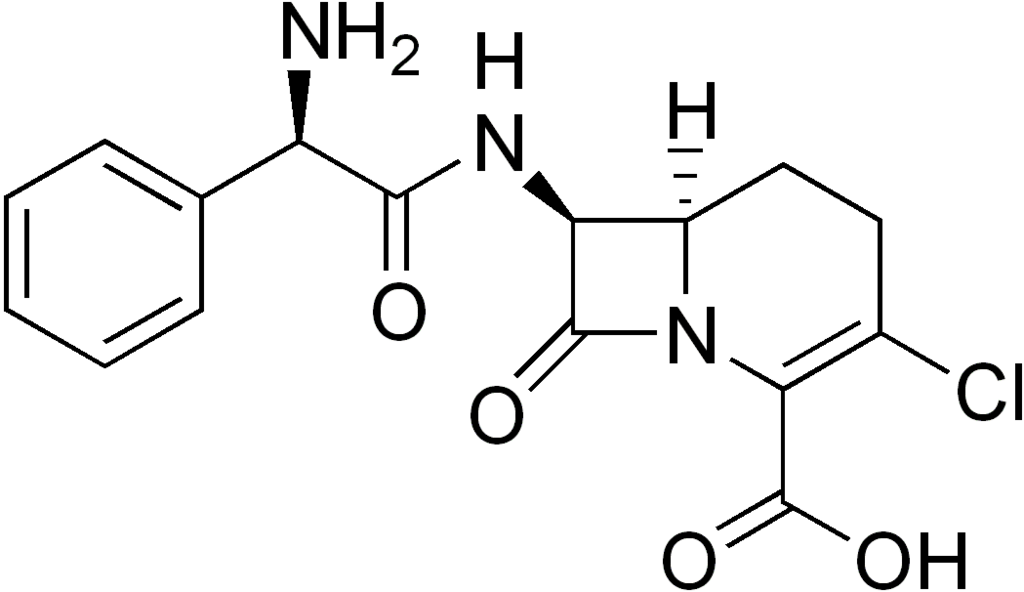
| IUPAC_name = (6''R'',7''S'')-7-<nowiki>[[</nowiki>(2''S'')-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |||
| image = Loracarbef.png | |||
<!--Clinical data--> | |||
| tradename = Lorabid | |||
| Drugs.com = {{drugs.com|monograph|loracarbef}} | |||
| MedlinePlus = a601206 | |||
| pregnancy_category = | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 25% | |||
| metabolism = | |||
| elimination_half-life = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|changed|??}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 76470-66-1 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = DC08 | |||
| PubChem = 5284584 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00447 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4447634 | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = W72I5ZT78Z | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08143 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1013 | |||
<!--Chemical data--> | |||
| C=16 | H=16 | Cl=1 | N=3 | O=4 | |||
| molecular_weight = 349.769 g/mol | |||
| smiles = Cl\C3=C(/C(=O)O)N2C(=O)[C@@H](NC(=O)[C@@H](c1ccccc1)N)[C@H]2CC3.O | |||
| InChI = 1/C16H16ClN3O4.H2O/c17-9-6-7-10-12(15(22)20(10)13(9)16(23)24)19-14(21)11(18)8-4-2-1-3-5-8;/h1-5,10-12H,6-7,18H2,(H,19,21)(H,23,24);1H2/t10-,11-,12+;/m1./s1 | |||
| InChIKey = GPYKKBAAPVOCIW-HSASPSRMBZ | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C16H16ClN3O4.H2O/c17-9-6-7-10-12(15(22)20(10)13(9)16(23)24)19-14(21)11(18)8-4-2-1-3-5-8;/h1-5,10-12H,6-7,18H2,(H,19,21)(H,23,24);1H2/t10-,11-,12+;/m1./s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}}= {{stdinchicite|correct|chemspider}}= {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = GPYKKBAAPVOCIW-HSASPSRMSA-N | |||
}} | |||
__NOTOC__ | __NOTOC__ | ||
{{CMG}} | |||
==Overview== | |||
Loracarbef is an [[antibiotic]].<ref name="pmid8150976">{{cite journal |author=Biedenbach DJ, Jones RN |title=Predictive accuracy of disk diffusion test for Proteus vulgaris and Providencia species against five newer orally administered cephalosporins, cefdinir, cefetamet, cefprozil, cefuroxime, and loracarbef |journal=J. Clin. Microbiol. |volume=32 |issue=2 |pages=559–62 |year=1994 |month=February |pmid=8150976 |pmc=263078 |doi= |url=http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=8150976}}</ref> Its use was discontinued in 2006.{{fact|date=January 2011}} It is a [[carbacephem]], but it is sometimes grouped together with the second-generation [[cephalosporin]] antibiotics. It was marketed under the trade name Lorabid. | |||
Loracarbef is a synthetic "carba" analogue of cefaclor, and is more stable chemically. Diarrhea is the most common adverse effect with Loracarbef. Side effects are more frequently seen with children under the age of twelve. It received FDA approval in 1991. | |||
==Category== | |||
Carbacephem | |||
==US Brand Names== | |||
LORABID<sup>®</sup> | |||
==Mechanism of Action== | |||
==References== | |||
{{Reflist|2}} | |||
==External links== | |||
* [http://www.rxlist.com/cgi/generic/loracarb.htm RxList.com - Loracarbef] | |||
[[Category:Antibiotics]] | |||
[[Category:Wikinfect]] | |||
Revision as of 03:19, 31 December 2013
 | |
| Clinical data | |
|---|---|
| Trade names | Lorabid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 25% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H16ClN3O4 |
| Molar mass | 349.769 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Loracarbef is an antibiotic.[1] Its use was discontinued in 2006.[citation needed] It is a carbacephem, but it is sometimes grouped together with the second-generation cephalosporin antibiotics. It was marketed under the trade name Lorabid.
Loracarbef is a synthetic "carba" analogue of cefaclor, and is more stable chemically. Diarrhea is the most common adverse effect with Loracarbef. Side effects are more frequently seen with children under the age of twelve. It received FDA approval in 1991.
Category
Carbacephem
US Brand Names
LORABID®
Mechanism of Action
References
- ↑ Biedenbach DJ, Jones RN (1994). "Predictive accuracy of disk diffusion test for Proteus vulgaris and Providencia species against five newer orally administered cephalosporins, cefdinir, cefetamet, cefprozil, cefuroxime, and loracarbef". J. Clin. Microbiol. 32 (2): 559–62. PMC 263078. PMID 8150976. Unknown parameter
|month=ignored (help)
External links
Categories:
- Pages with script errors
- Pages with citations using unsupported parameters
- Template:drugs.com link with non-standard subpage
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- All articles with unsourced statements
- Articles with unsourced statements from January 2011
- Articles with invalid date parameter in template
- Antibiotics
- Wikinfect