Lincomycin labels and packages
| Lincomycin |
|---|
| LINCOCIN®, LINCOMED®, LINCOMIX® FDA Package Insert |
| Description |
| Clinical Pharmacology |
| Microbiology |
| Indications and Usage |
| Contraindications |
| Warnings |
| Precautions |
| Adverse Reactions |
| Overdosage |
| Dosage and Administration |
| How Supplied |
| Compatiblity |
| Labels and Packages |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
[image:Pfizer.jpg]]
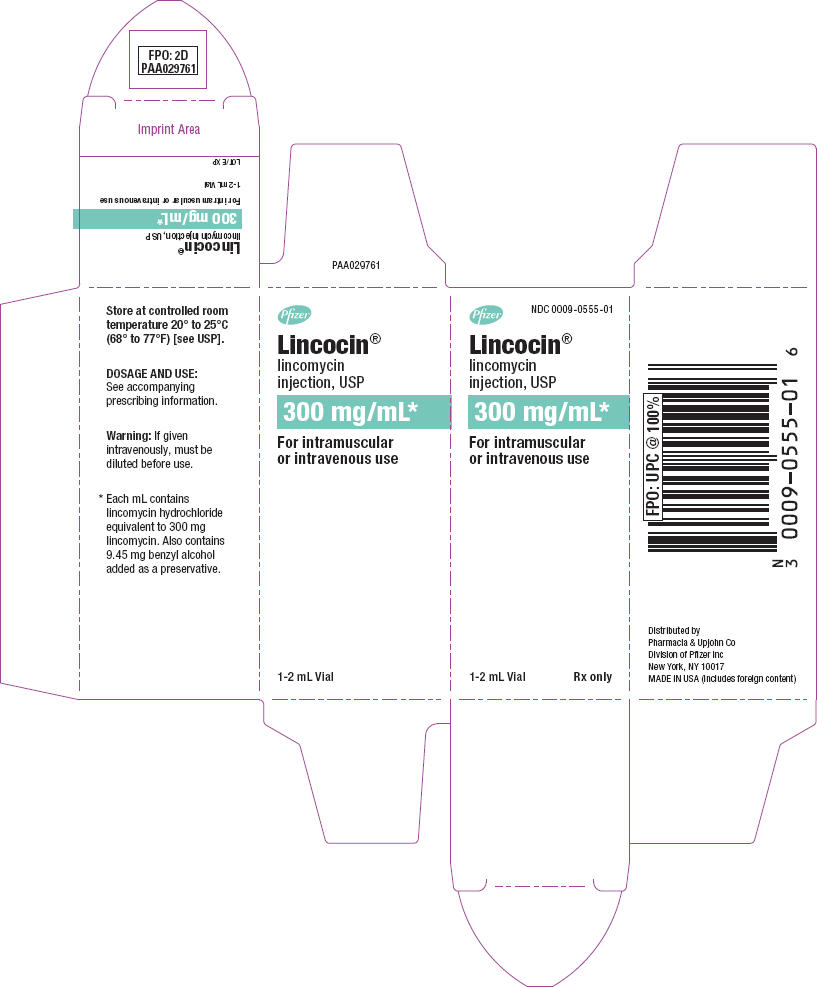
PRINCIPAL DISPLAY PANEL - 1-2 mL Vial Label
Pfizer
NDC 0009-0555-01
Lincocin® lincomycin injection, USP
300 mg/mL
1-2 mL Vial Rx only
 |
PRINCIPAL DISPLAY PANEL - 1-2 mL Vial Carton
Pfizer NDC 0009-0555-01
Lincocin® lincomycin injection, USP
300 mg/mL*
For intramuscular or intravenous use
1-2 mL Vial Rx only
 |
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050317s172lbl.pdf