Levonorgestrel (oral)
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Implant; insert (extended-release); oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 55% |
| Metabolism | Hepatic |
| Elimination half-life | 36 ± 13 hours |
| Excretion | Renal: 45%; Fecal:32% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
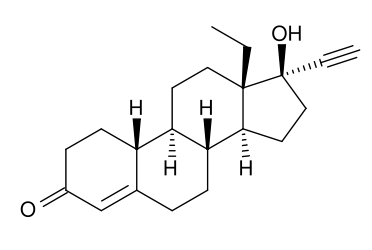
| Formula | C21H28O2 |
| Molar mass | 312.446 g/mol |
Levonorgestrel (or l-norgestrel or D-norgestrel) is a synthetic progestogen used as an active ingredient in some hormonal contraceptives.
Chemistry
Chemically, it is a hormonally active levorotatory enantiomer of the racemic mixture norgestrel. It is a gonane progestin derived from 19-nortestosterone.[1]
Its in vitro relative binding affinities at human steroid hormone receptors are: 323% that of progesterone at the progesterone receptor, 58% that of testosterone at the androgen receptor, 17% that of aldosterone at the mineralocorticoid receptor, 7.5% that of cortisol at the glucocorticoid receptor, and <0.02% that of estradiol at the estrogen receptor.[2]
Usage
Oral contraceptives
At low doses, levonorgestrel is used in monophasic and triphasic formulations of combined oral contraceptive pills, with available monophasic doses ranging from 100-250 µg, and triphasic doses of 50 µg/75 µg/125 µg.
At very low daily dose of 30 µg, levonorgestrel is used in some progestogen only pill formulations.
Emergency contraception
Levonorgestrel is used in emergency contraceptive pills (ECPs), both in a combined Yuzpe regimen which includes estrogen, and as a levonorgestrel-only method. The levonorgestrel-only method uses levonorgestrel 1500 μg (as a single dose or as two 750 μg doses 12 hours apart) taken within 3 days of unprotected sex. There are many brand names of levonorgestrel-only ECPs, including: Plan B, Levonelle, NorLevo, Postinor-2, and 72-HOURS.[3]
IUD
Levonorgestrel is the active ingredient in Mirena.
Contraceptive implants
Levonorgestrel is the active ingredient in Norplant and Jadelle.
References
- ↑ Edgren RA, Stanczyk FZ (1999). "Nomenclature of the gonane progestins". Contraception. 60 (6): 313. PMID 10715364.
- ↑ Sitruk-Ware R (2006). "New progestagens for contraceptive use". Hum Reprod Update. 12 (2): 169–78. PMID 16291771.
- ↑ Trussell, James; Cleland, Kelly (2007-04-10). "Emergency Contraceptive Pills Worldwide". Princeton University. Retrieved 2007-05-28.
External links
- Levonelle manufacturer's product information from Schering
- Monograph for levonorgestrel - Uk Medicines Information
Template:Sex hormones Template:SIB de:Levonorgestrel nl:Levonorgestrel
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Hormonal contraception
- Progestagens
- Endocrinology