Leflunomide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 412: | Line 412: | ||
|structure= | |structure= | ||
* | * Leflunomide is a pyrimidine synthesis inhibitor. The chemical name for leflunomide is N-(4'-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide. It has an empirical formula C12H9F3N2O2, a molecular weight of 270.2 and the following structural formula: | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Leflunomide tablets, USP is available for oral administration as tablets containing 10 or 20 mg of active drug. Each leflunomide tablet, USP contains anhydrous lactose, colloidal silicon dioxide, crospovidone, and magnesium stearate. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 426: | Line 428: | ||
|PK= | |PK= | ||
*Following oral administration, leflunomide is metabolized to an active metabolite A77 1726 (hereafter referred to as M1) which is responsible for essentially all of its activity in vivo. Plasma levels of leflunomide are occasionally seen, at very low levels. Studies of the pharmacokinetics of leflunomide have primarily examined the plasma concentrations of this active metabolite. | |||
*Absorption | |||
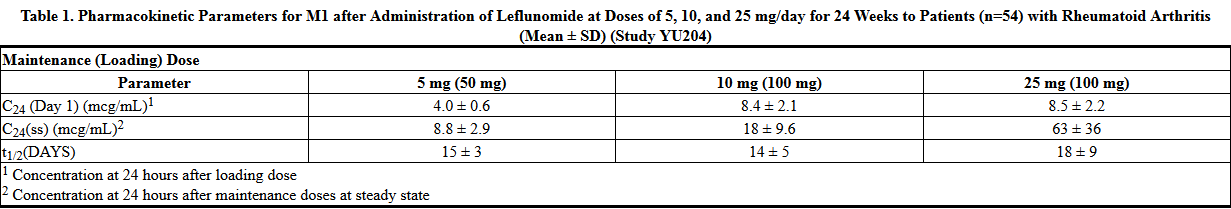
:*Following oral administration, peak levels of the active metabolite, M1, occurred between 6 to 12 hours after dosing. Due to the very long half-life of M1 (~2 weeks), a loading dose of 100 mg for 3 days was used in clinical studies to facilitate the rapid attainment of steady-state levels of M1. Without a loading dose, it is estimated that attainment of steady-state plasma concentrations would require nearly two months of dosing. The resulting plasma concentrations following both loading doses and continued clinical dosing indicate that M1 plasma levels are dose proportional. | |||
T1 | |||
:*Relative to an oral solution, leflunomide tablets are 80% bioavailable. Co-administration of leflunomide tablets with a high fat meal did not have a significant impact on M1 plasma levels. | |||
*Distribution | |||
:*M1 has a low volume of distribution (Vss = 0.13 L/kg) and is extensively bound (>99.3%) to albumin in healthy subjects. Protein binding has been shown to be linear at therapeutic concentrations. The free fraction of M1 is slightly higher in patients with rheumatoid arthritis and approximately doubled in patients with chronic renal failure; the mechanism and significance of these increases are unknown. | |||
*Metabolism | |||
:*Leflunomide is metabolized to one primary (M1) and many minor metabolites. Of these minor metabolites, only 4-trifluoromethylaniline (TFMA) is quantifiable, occurring at low levels in the plasma of some patients. The parent compound is rarely detectable in plasma. At the present time the specific site of leflunomide metabolism is unknown. In vivo and in vitro studies suggest a role for both the GI wall and the liver in drug metabolism. No specific enzyme has been identified as the primary route of metabolism for leflunomide; however, hepatic cytosolic and microsomal cellular fractions have been identified as sites of drug metabolism. | |||
*Elimination | |||
:*The active metabolite M1 is eliminated by further metabolism and subsequent renal excretion as well as by direct biliary excretion. In a 28 day study of drug elimination (n=3) using a single dose of radiolabeled compound, approximately 43% of the total radioactivity was eliminated in the urine and 48% was eliminated in the feces. Subsequent analysis of the samples revealed the primary urinary metabolites to be leflunomide glucuronides and an oxanilic acid derivative of M1. The primary fecal metabolite was M1. Of these two routes of elimination, renal elimination is more significant over the first 96 hours after which fecal elimination begins to predominate. In a study involving the intravenous administration of M1, the clearance was estimated to be 31 mL/hr. | |||
:*In small studies using activated charcoal (n=1) or cholestyramine (n=3) to facilitate drug elimination, the in vivo plasma half-life of M1 was reduced from >1 week to approximately 1 day (see PRECAUTIONS, General, Need for Drug Elimination), Similar reductions in plasma half-life were observed for a series of volunteers (n=96) enrolled in pharmacokinetic trials who were given cholestyramine. This suggests that biliary recycling is a major contributor to the long elimination half-life of M1. Studies with both hemodialysis and CAPD (chronic ambulatory peritoneal dialysis) indicate that M1 is not dialyzable. | |||
*Special Populations | |||
*Gender | |||
:*Gender has not been shown to cause a consistent change in the in vivo pharmacokinetics of M1. | |||
*Age | |||
:*Age has been shown to cause a change in the in vivo pharmacokinetics of M1 (see CLINICAL PHARMACOLOGY, Special Populations, Pediatrics). | |||
*Smoking | |||
:*A population based pharmacokinetic analysis of the phase III data indicates that smokers have a 38% increase in clearance over non-smokers; however, no difference in clinical efficacy was seen between smokers and nonsmokers. | |||
*Chronic Renal Insufficiency | |||
:*In single dose studies in patients (n=6) with chronic renal insufficiency requiring either chronic ambulatory peritoneal dialysis (CAPD) or hemodialysis, neither had a significant impact on circulating levels of M1. The free fraction of M1 was almost doubled, but the mechanism of this increase is not known. In light of the fact that the kidney plays a role in drug elimination and without adequate studies of leflunomide use in subjects with renal insufficiency, caution should be used when leflunomide is administered to these patients. | |||
*Hepatic Insufficiency | |||
:*Studies of the effect of hepatic insufficiency on M1 pharmacokinetics have not been done. Given the need to metabolize leflunomide into the active species, the role of the liver in drug elimination/recycling, and the possible risk of increased hepatic toxicity, the use of leflunomide in patients with hepatic insufficiency is not recommended. | |||
*Pediatrics | |||
:*The pharmacokinetics of M1 following oral administration of leflunomide have been investigated in 73 pediatric patients with polyarticular course Juvenile Rheumatoid Arthritis (JRA) who ranged in age from 3 to 17 years. The results of a population pharmacokinetic analysis of these trials have demonstrated that pediatric patients with body weights ≤40 kg have a reduced clearance of M1 (see Table 2) relative to adult rheumatoid arthritis patients. | |||
T2 | |||
*Drug Interactions | |||
:*In vivo drug interaction studies have demonstrated a lack of a significant drug interaction between leflunomide and tri-phasic oral contraceptives, and cimetidine. | |||
:*In vitro studies of protein binding indicated that warfarin did not affect M1 protein binding. At the same time M1 was shown to cause increases ranging from 13 to 50% in the free fraction of diclofenac, ibuprofen and tolbutamide at concentrations in the clinical range. In vitro studies of drug metabolism indicate that M1 inhibits CYP 450 2C9, which is responsible for the metabolism of phenytoin, tolbutamide, warfarin and many NSAIDs. M1 has been shown to inhibit the formation of 4′-hydroxydiclofenac from diclofenac in vitro. The clinical significance of these findings with regard to phenytoin and tolbutamide is unknown; however, there was extensive concomitant use of NSAIDs in the clinical studies and no differential effect was observed (see PRECAUTIONS, Drug Interactions). | |||
*Methotrexate | |||
:*Coadministration, in 30 patients, of leflunomide (100 mg/day x 2 days followed by 10 to 20 mg/day) with methotrexate (10 to 25 mg/week, with folate) demonstrated no pharmacokinetic interaction between the two drugs. However, co-administration increased risk of hepatotoxicity (see PRECAUTIONS, Drug Interactions, Hepatotoxic Drugs). | |||
*Rifampin | |||
:*Following concomitant administration of a single dose of leflunomide to subjects receiving multiple doses of rifampin, M1 peak levels were increased (~40%) over those seen when leflunomide was given alone. Because of the potential for leflunomide levels to continue to increase with multiple dosing, caution should be used if patients are to receive both leflunomide and rifampin. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
| Line 438: | Line 489: | ||
|clinicalStudies= | |clinicalStudies= | ||
There is | =====ADULTS===== | ||
*The efficacy of leflunomide in the treatment of rheumatoid arthritis (RA) was demonstrated in three controlled trials showing reduction in signs and symptoms, and inhibition of structural damage. In two placebo controlled trials, efficacy was demonstrated for improvement in physical function. | |||
*Reduction of signs and symptoms | |||
:*Relief of signs and symptoms was assessed using the American College of Rheumatology (ACR) 20 Responder Index, a composite of clinical, laboratory, and functional measures in rheumatoid arthritis. An "ACR20 Responder" is a patient who had ≥ 20% improvement in both tender and swollen joint counts and in 3 of the following 5 criteria: physician global assessment, patient global assessment, functional ability measure [Modified Health Assessment Questionnaire (MHAQ)], visual analog pain scale, and erythrocyte sedimentation rate or C-reactive protein. An "ACR20 Responder at Endpoint" is a patient who completed the study and was an ACR20 Responder at the completion of the study. | |||
*Inhibition of structural damage | |||
:*Inhibition of structural damage compared to control was assessed using the Sharp Score (Sharp, JT. Scoring Radiographic Abnormalities in Rheumatoid Arthritis, Radiologic Clinics of North America, 1996; vol. 34, pp. 233 to 241), a composite score of X-ray erosions and joint space narrowing in hands/wrists and forefeet. | |||
*Improvement in physical function | |||
:*Improvement in physical function was assessed using the Health Assessment Questionnaire (HAQ) and the Medical Outcomes Survey Short Form (SF-36). | |||
:*In all leflunomide monotherapy studies, an initial loading dose of 100 mg per day for three days only was used followed by 20 mg per day thereafter. | |||
*US301 Clinical Trial in Adults | |||
:*Study US301, a 2 year study, randomized 482 patients with active RA of at least 6 months duration to leflunomide 20 mg/day (n=182), methotrexate 7.5 mg/week increasing to 15 mg/week (n=182), or placebo (n=118). All patients received folate 1 mg BID. Primary analysis was at 52 weeks with blinded treatment to 104 weeks. | |||
:*Overall, 235 of the 508 randomized treated patients (482 in primary data analysis and an additional 26 patients), continued into a second 12 months of double-blind treatment (98 leflunomide, 101 methotrexate, 36 placebo). Leflunomide dose continued at 20 mg/day and the methotrexate dose could be increased to a maximum of 20 mg/week. In total, 190 patients (83 leflunomide, 80 methotrexate, 27 placebo) completed 2 years of double-blind treatment. | |||
:*The rate and reason for withdrawal is summarized in Table 3. | |||
T3 | |||
:*1 Includes: lost to follow up, protocol violation, noncompliance, voluntary withdrawal, investigator discretion. | |||
*MN301/303/305 Clinical Trial in Adults | |||
:*Study MN301 randomized 358 patients with active RA to leflunomide 20 mg/day (n=133), sulfasalazine 2.0 g/day (n=133), or placebo (n=92). Treatment duration was 24 weeks. An extension of the study was an optional 6-month blinded continuation of MN301 without the placebo arm, resulting in a 12-month comparison of leflunomide and sulfasalazine (study MN303). | |||
:*Of the 168 patients who completed 12 months of treatment in MN301 and MN303, 146 patients (87%) entered a 1-year extension study of double blind active treatment (MN305; 60 leflunomide, 60 sulfasalazine, 26 placebo/sulfasalazine). Patients continued on the same daily dosage of leflunomide or sulfasalazine that they had been taking at the completion of MN301/303. A total of 121 patients (53 leflunomide, 47 sulfasalazine, 21 placebo/sulfasalazine) completed the 2 years of double-blind treatment. | |||
:*Patient withdrawal data in MN301/303/305 is summarized in Table 4. | |||
T4 | |||
*MN302/304 Clinical Trial in Adults | |||
:*Study MN302 randomized 999 patients with active RA to leflunomide 20 mg/day (n=501) or methotrexate at 7.5 mg/week increasing to 15 mg/week (n=498). Folate supplementation was used in 10% of patients. Treatment duration was 52 weeks. | |||
:*Of the 736 patients who completed 52 weeks of treatment in study MN302, 612 (83%) entered the double-blind, 1-year extension study MN304 (292 leflunomide, 320 methotrexate). Patients continued on the same daily dosage of leflunomide or methotrexate that they had been taking at the completion of MN302. There were 533 patients (256 leflunomide, 277 methotrexate) who completed 2 years of double-blind treatment. | |||
:*Patient withdrawal data in MN302/304 is summarized in Table 5. | |||
T5 | |||
=====Clinical Trial Data===== | |||
*Signs and symptoms Rheumatoid Arthritis | |||
:*The ACR20 Responder at Endpoint rates are shown in Figure 1. Leflunomide was statistically significantly superior to placebo in reducing the signs and symptoms of RA by the primary efficacy analysis, ACR20 Responder at Endpoint, in study US301 (at the primary 12 months endpoint) and MN301 (at 6 month endpoint). ACR20 Responder at Endpoint rates with leflunomide treatment were consistent across the 6 and 12 month studies (41 to 49%). No consistent differences were demonstrated between leflunomide and methotrexate or between leflunomide and sulfasalazine. Leflunomide treatment effect was evident by 1 month, stabilized by 3 to 6 months, and continued throughout the course of treatment as shown in Figure 2. | |||
Figure 1 | |||
T | |||
F2 | |||
:*ACR50 and ACR70 Responders are defined in an analogous manner to the ACR20 Responder, but use improvements of 50% or 70%, respectively (Table 6). Mean change for the individual components of the ACR Responder Index are shown in Table 7. | |||
T6 | |||
:*Table 7 shows the results of the components of the ACR response criteria for US301, MN301, and MN302. Leflunomide was significantly superior to placebo in all components of the ACR Response criteria in study US301 and MN301. In addition, leflunomide was significantly superior to placebo in improving morning stiffness, a measure of RA disease activity, not included in the ACR Response criteria. No consistent differences were demonstrated between leflunomide and the active comparators. | |||
T7 | |||
*Maintenance of effect | |||
:*After completing 12 months of treatment, patients continuing on study treatment were evaluated for an additional 12 months of double-blind treatment (total treatment period of 2 years) in studies US301, MN305, and MN304. ACR Responder rates at 12 months were maintained over 2 years in most patients continuing a second year of treatment. | |||
:*Improvement from baseline in the individual components of the ACR responder criteria was also sustained in most patients during the second year of leflunomide treatment in all three trials. | |||
*Inhibition of structural damage | |||
:*The change from baseline to endpoint in progression of structural disease, as measured by the Sharp X-ray score, is displayed in Figure 3. Leflunomide was statistically significantly superior to placebo in inhibiting the progression of disease by the Sharp Score. No consistent differences were demonstrated between leflunomide and methotrexate or between leflunomide and sulfasalazine. | |||
Figure 3 | |||
T | |||
*Improvement in physical function | |||
:*The Health Assessment Questionnaire (HAQ) assesses a patient's physical function and degree of disability. The mean change from baseline in functional ability as measured by the HAQ Disability Index (HAQ DI) in the 6 and 12 month placebo and active controlled trials is shown in Figure 4. Leflunomide was statistically significantly superior to placebo in improving physical function. Superiority to placebo was demonstrated consistently across all eight HAQ DI subscales (dressing, arising, eating, walking, hygiene, reach, grip and activities) in both placebo controlled studies. | |||
:*The Medical Outcomes Survey Short Form 36 (SF-36), a generic health-related quality of life questionnaire, further addresses physical function. In US301, at 12 months, leflunomide provided statistically significant improvements compared to placebo in the Physical Component Summary (PCS) Score. | |||
Figure 4 | |||
T | |||
*Maintenance of effect | |||
:*The improvement in physical function demonstrated at 6 and 12 months was maintained over two years. In those patients continuing therapy for a second year, this improvement in physical function as measured by HAQ and SF-36 (PCS) was maintained. | |||
=====PEDIATRICS===== | |||
*Clinical Trials in Pediatrics | |||
:*Leflunomide was studied in a single multicenter, double-blind, active-controlled trial in 94 patients (1:1 randomization) with polyarticular course juvenile rheumatoid arthritis (JRA) as defined by the American College of Rheumatology (ACR). Approximately 68% of pediatric patients receiving leflunomide, versus 89% of pediatric patients receiving the active comparator, improved by Week 16 (end-of-study) employing the JRA Definition of Improvement (DOI) ≥ 30 % responder endpoint. In this trial, the loading dose and maintenance dose of leflunomide was based on three weight categories: <20 kg, 20 to 40kg, and >40 kg. The response rate to leflunomide in pediatric patients ≤40 kg was less robust than in pediatric patients >40 kg suggesting suboptimal dosing in smaller weight pediatric patients, as studied, resulting in less than efficacious plasma concentrations, despite reduced clearance of M1. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
Revision as of 16:28, 28 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
PREGNANCY AND HEPATOTOXICITY
See full prescribing information for complete Boxed Warning.
|
Overview
Leflunomide is a pyrimidine synthesis inhibitor that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Leflunomide in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Leflunomide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Leflunomide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Leflunomide in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Leflunomide in pediatric patients.
Contraindications
- Condition1
Warnings
|
PREGNANCY AND HEPATOTOXICITY
See full prescribing information for complete Boxed Warning.
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Leflunomide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Leflunomide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Leflunomide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Leflunomide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Leflunomide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Leflunomide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Leflunomide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Leflunomide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Leflunomide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Leflunomide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Leflunomide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Leflunomide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Leflunomide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Leflunomide in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Leflunomide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Leflunomide in the drug label.
Pharmacology
There is limited information regarding Leflunomide Pharmacology in the drug label.
Mechanism of Action
- Leflunomide is an isoxazole immunomodulatory agent which inhibits dihydroorotate dehydrogenase (an enzyme involved in de novo pyrimidine synthesis) and has antiproliferative activity. Several in vivo and in vitro experimental models have demonstrated an anti-inflammatory effect.
Structure
- Leflunomide is a pyrimidine synthesis inhibitor. The chemical name for leflunomide is N-(4'-trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide. It has an empirical formula C12H9F3N2O2, a molecular weight of 270.2 and the following structural formula:
- Leflunomide tablets, USP is available for oral administration as tablets containing 10 or 20 mg of active drug. Each leflunomide tablet, USP contains anhydrous lactose, colloidal silicon dioxide, crospovidone, and magnesium stearate.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Leflunomide in the drug label.
Pharmacokinetics
- Following oral administration, leflunomide is metabolized to an active metabolite A77 1726 (hereafter referred to as M1) which is responsible for essentially all of its activity in vivo. Plasma levels of leflunomide are occasionally seen, at very low levels. Studies of the pharmacokinetics of leflunomide have primarily examined the plasma concentrations of this active metabolite.
- Absorption
- Following oral administration, peak levels of the active metabolite, M1, occurred between 6 to 12 hours after dosing. Due to the very long half-life of M1 (~2 weeks), a loading dose of 100 mg for 3 days was used in clinical studies to facilitate the rapid attainment of steady-state levels of M1. Without a loading dose, it is estimated that attainment of steady-state plasma concentrations would require nearly two months of dosing. The resulting plasma concentrations following both loading doses and continued clinical dosing indicate that M1 plasma levels are dose proportional.
T1
- Relative to an oral solution, leflunomide tablets are 80% bioavailable. Co-administration of leflunomide tablets with a high fat meal did not have a significant impact on M1 plasma levels.
- Distribution
- M1 has a low volume of distribution (Vss = 0.13 L/kg) and is extensively bound (>99.3%) to albumin in healthy subjects. Protein binding has been shown to be linear at therapeutic concentrations. The free fraction of M1 is slightly higher in patients with rheumatoid arthritis and approximately doubled in patients with chronic renal failure; the mechanism and significance of these increases are unknown.
- Metabolism
- Leflunomide is metabolized to one primary (M1) and many minor metabolites. Of these minor metabolites, only 4-trifluoromethylaniline (TFMA) is quantifiable, occurring at low levels in the plasma of some patients. The parent compound is rarely detectable in plasma. At the present time the specific site of leflunomide metabolism is unknown. In vivo and in vitro studies suggest a role for both the GI wall and the liver in drug metabolism. No specific enzyme has been identified as the primary route of metabolism for leflunomide; however, hepatic cytosolic and microsomal cellular fractions have been identified as sites of drug metabolism.
- Elimination
- The active metabolite M1 is eliminated by further metabolism and subsequent renal excretion as well as by direct biliary excretion. In a 28 day study of drug elimination (n=3) using a single dose of radiolabeled compound, approximately 43% of the total radioactivity was eliminated in the urine and 48% was eliminated in the feces. Subsequent analysis of the samples revealed the primary urinary metabolites to be leflunomide glucuronides and an oxanilic acid derivative of M1. The primary fecal metabolite was M1. Of these two routes of elimination, renal elimination is more significant over the first 96 hours after which fecal elimination begins to predominate. In a study involving the intravenous administration of M1, the clearance was estimated to be 31 mL/hr.
- In small studies using activated charcoal (n=1) or cholestyramine (n=3) to facilitate drug elimination, the in vivo plasma half-life of M1 was reduced from >1 week to approximately 1 day (see PRECAUTIONS, General, Need for Drug Elimination), Similar reductions in plasma half-life were observed for a series of volunteers (n=96) enrolled in pharmacokinetic trials who were given cholestyramine. This suggests that biliary recycling is a major contributor to the long elimination half-life of M1. Studies with both hemodialysis and CAPD (chronic ambulatory peritoneal dialysis) indicate that M1 is not dialyzable.
- Special Populations
- Gender
- Gender has not been shown to cause a consistent change in the in vivo pharmacokinetics of M1.
- Age
- Age has been shown to cause a change in the in vivo pharmacokinetics of M1 (see CLINICAL PHARMACOLOGY, Special Populations, Pediatrics).
- Smoking
- A population based pharmacokinetic analysis of the phase III data indicates that smokers have a 38% increase in clearance over non-smokers; however, no difference in clinical efficacy was seen between smokers and nonsmokers.
- Chronic Renal Insufficiency
- In single dose studies in patients (n=6) with chronic renal insufficiency requiring either chronic ambulatory peritoneal dialysis (CAPD) or hemodialysis, neither had a significant impact on circulating levels of M1. The free fraction of M1 was almost doubled, but the mechanism of this increase is not known. In light of the fact that the kidney plays a role in drug elimination and without adequate studies of leflunomide use in subjects with renal insufficiency, caution should be used when leflunomide is administered to these patients.
- Hepatic Insufficiency
- Studies of the effect of hepatic insufficiency on M1 pharmacokinetics have not been done. Given the need to metabolize leflunomide into the active species, the role of the liver in drug elimination/recycling, and the possible risk of increased hepatic toxicity, the use of leflunomide in patients with hepatic insufficiency is not recommended.
- Pediatrics
- The pharmacokinetics of M1 following oral administration of leflunomide have been investigated in 73 pediatric patients with polyarticular course Juvenile Rheumatoid Arthritis (JRA) who ranged in age from 3 to 17 years. The results of a population pharmacokinetic analysis of these trials have demonstrated that pediatric patients with body weights ≤40 kg have a reduced clearance of M1 (see Table 2) relative to adult rheumatoid arthritis patients.
T2
- Drug Interactions
- In vivo drug interaction studies have demonstrated a lack of a significant drug interaction between leflunomide and tri-phasic oral contraceptives, and cimetidine.
- In vitro studies of protein binding indicated that warfarin did not affect M1 protein binding. At the same time M1 was shown to cause increases ranging from 13 to 50% in the free fraction of diclofenac, ibuprofen and tolbutamide at concentrations in the clinical range. In vitro studies of drug metabolism indicate that M1 inhibits CYP 450 2C9, which is responsible for the metabolism of phenytoin, tolbutamide, warfarin and many NSAIDs. M1 has been shown to inhibit the formation of 4′-hydroxydiclofenac from diclofenac in vitro. The clinical significance of these findings with regard to phenytoin and tolbutamide is unknown; however, there was extensive concomitant use of NSAIDs in the clinical studies and no differential effect was observed (see PRECAUTIONS, Drug Interactions).
- Methotrexate
- Coadministration, in 30 patients, of leflunomide (100 mg/day x 2 days followed by 10 to 20 mg/day) with methotrexate (10 to 25 mg/week, with folate) demonstrated no pharmacokinetic interaction between the two drugs. However, co-administration increased risk of hepatotoxicity (see PRECAUTIONS, Drug Interactions, Hepatotoxic Drugs).
- Rifampin
- Following concomitant administration of a single dose of leflunomide to subjects receiving multiple doses of rifampin, M1 peak levels were increased (~40%) over those seen when leflunomide was given alone. Because of the potential for leflunomide levels to continue to increase with multiple dosing, caution should be used if patients are to receive both leflunomide and rifampin.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Leflunomide in the drug label.
Clinical Studies
ADULTS
- The efficacy of leflunomide in the treatment of rheumatoid arthritis (RA) was demonstrated in three controlled trials showing reduction in signs and symptoms, and inhibition of structural damage. In two placebo controlled trials, efficacy was demonstrated for improvement in physical function.
- Reduction of signs and symptoms
- Relief of signs and symptoms was assessed using the American College of Rheumatology (ACR) 20 Responder Index, a composite of clinical, laboratory, and functional measures in rheumatoid arthritis. An "ACR20 Responder" is a patient who had ≥ 20% improvement in both tender and swollen joint counts and in 3 of the following 5 criteria: physician global assessment, patient global assessment, functional ability measure [Modified Health Assessment Questionnaire (MHAQ)], visual analog pain scale, and erythrocyte sedimentation rate or C-reactive protein. An "ACR20 Responder at Endpoint" is a patient who completed the study and was an ACR20 Responder at the completion of the study.
- Inhibition of structural damage
- Inhibition of structural damage compared to control was assessed using the Sharp Score (Sharp, JT. Scoring Radiographic Abnormalities in Rheumatoid Arthritis, Radiologic Clinics of North America, 1996; vol. 34, pp. 233 to 241), a composite score of X-ray erosions and joint space narrowing in hands/wrists and forefeet.
- Improvement in physical function
- Improvement in physical function was assessed using the Health Assessment Questionnaire (HAQ) and the Medical Outcomes Survey Short Form (SF-36).
- In all leflunomide monotherapy studies, an initial loading dose of 100 mg per day for three days only was used followed by 20 mg per day thereafter.
- US301 Clinical Trial in Adults
- Study US301, a 2 year study, randomized 482 patients with active RA of at least 6 months duration to leflunomide 20 mg/day (n=182), methotrexate 7.5 mg/week increasing to 15 mg/week (n=182), or placebo (n=118). All patients received folate 1 mg BID. Primary analysis was at 52 weeks with blinded treatment to 104 weeks.
- Overall, 235 of the 508 randomized treated patients (482 in primary data analysis and an additional 26 patients), continued into a second 12 months of double-blind treatment (98 leflunomide, 101 methotrexate, 36 placebo). Leflunomide dose continued at 20 mg/day and the methotrexate dose could be increased to a maximum of 20 mg/week. In total, 190 patients (83 leflunomide, 80 methotrexate, 27 placebo) completed 2 years of double-blind treatment.
- The rate and reason for withdrawal is summarized in Table 3.
T3
- 1 Includes: lost to follow up, protocol violation, noncompliance, voluntary withdrawal, investigator discretion.
- MN301/303/305 Clinical Trial in Adults
- Study MN301 randomized 358 patients with active RA to leflunomide 20 mg/day (n=133), sulfasalazine 2.0 g/day (n=133), or placebo (n=92). Treatment duration was 24 weeks. An extension of the study was an optional 6-month blinded continuation of MN301 without the placebo arm, resulting in a 12-month comparison of leflunomide and sulfasalazine (study MN303).
- Of the 168 patients who completed 12 months of treatment in MN301 and MN303, 146 patients (87%) entered a 1-year extension study of double blind active treatment (MN305; 60 leflunomide, 60 sulfasalazine, 26 placebo/sulfasalazine). Patients continued on the same daily dosage of leflunomide or sulfasalazine that they had been taking at the completion of MN301/303. A total of 121 patients (53 leflunomide, 47 sulfasalazine, 21 placebo/sulfasalazine) completed the 2 years of double-blind treatment.
- Patient withdrawal data in MN301/303/305 is summarized in Table 4.
T4
- MN302/304 Clinical Trial in Adults
- Study MN302 randomized 999 patients with active RA to leflunomide 20 mg/day (n=501) or methotrexate at 7.5 mg/week increasing to 15 mg/week (n=498). Folate supplementation was used in 10% of patients. Treatment duration was 52 weeks.
- Of the 736 patients who completed 52 weeks of treatment in study MN302, 612 (83%) entered the double-blind, 1-year extension study MN304 (292 leflunomide, 320 methotrexate). Patients continued on the same daily dosage of leflunomide or methotrexate that they had been taking at the completion of MN302. There were 533 patients (256 leflunomide, 277 methotrexate) who completed 2 years of double-blind treatment.
- Patient withdrawal data in MN302/304 is summarized in Table 5.
T5
Clinical Trial Data
- Signs and symptoms Rheumatoid Arthritis
- The ACR20 Responder at Endpoint rates are shown in Figure 1. Leflunomide was statistically significantly superior to placebo in reducing the signs and symptoms of RA by the primary efficacy analysis, ACR20 Responder at Endpoint, in study US301 (at the primary 12 months endpoint) and MN301 (at 6 month endpoint). ACR20 Responder at Endpoint rates with leflunomide treatment were consistent across the 6 and 12 month studies (41 to 49%). No consistent differences were demonstrated between leflunomide and methotrexate or between leflunomide and sulfasalazine. Leflunomide treatment effect was evident by 1 month, stabilized by 3 to 6 months, and continued throughout the course of treatment as shown in Figure 2.
Figure 1
T
F2
- ACR50 and ACR70 Responders are defined in an analogous manner to the ACR20 Responder, but use improvements of 50% or 70%, respectively (Table 6). Mean change for the individual components of the ACR Responder Index are shown in Table 7.
T6
- Table 7 shows the results of the components of the ACR response criteria for US301, MN301, and MN302. Leflunomide was significantly superior to placebo in all components of the ACR Response criteria in study US301 and MN301. In addition, leflunomide was significantly superior to placebo in improving morning stiffness, a measure of RA disease activity, not included in the ACR Response criteria. No consistent differences were demonstrated between leflunomide and the active comparators.
T7
- Maintenance of effect
- After completing 12 months of treatment, patients continuing on study treatment were evaluated for an additional 12 months of double-blind treatment (total treatment period of 2 years) in studies US301, MN305, and MN304. ACR Responder rates at 12 months were maintained over 2 years in most patients continuing a second year of treatment.
- Improvement from baseline in the individual components of the ACR responder criteria was also sustained in most patients during the second year of leflunomide treatment in all three trials.
- Inhibition of structural damage
- The change from baseline to endpoint in progression of structural disease, as measured by the Sharp X-ray score, is displayed in Figure 3. Leflunomide was statistically significantly superior to placebo in inhibiting the progression of disease by the Sharp Score. No consistent differences were demonstrated between leflunomide and methotrexate or between leflunomide and sulfasalazine.
Figure 3
T
- Improvement in physical function
- The Health Assessment Questionnaire (HAQ) assesses a patient's physical function and degree of disability. The mean change from baseline in functional ability as measured by the HAQ Disability Index (HAQ DI) in the 6 and 12 month placebo and active controlled trials is shown in Figure 4. Leflunomide was statistically significantly superior to placebo in improving physical function. Superiority to placebo was demonstrated consistently across all eight HAQ DI subscales (dressing, arising, eating, walking, hygiene, reach, grip and activities) in both placebo controlled studies.
- The Medical Outcomes Survey Short Form 36 (SF-36), a generic health-related quality of life questionnaire, further addresses physical function. In US301, at 12 months, leflunomide provided statistically significant improvements compared to placebo in the Physical Component Summary (PCS) Score.
Figure 4
T
- Maintenance of effect
- The improvement in physical function demonstrated at 6 and 12 months was maintained over two years. In those patients continuing therapy for a second year, this improvement in physical function as measured by HAQ and SF-36 (PCS) was maintained.
PEDIATRICS
- Clinical Trials in Pediatrics
- Leflunomide was studied in a single multicenter, double-blind, active-controlled trial in 94 patients (1:1 randomization) with polyarticular course juvenile rheumatoid arthritis (JRA) as defined by the American College of Rheumatology (ACR). Approximately 68% of pediatric patients receiving leflunomide, versus 89% of pediatric patients receiving the active comparator, improved by Week 16 (end-of-study) employing the JRA Definition of Improvement (DOI) ≥ 30 % responder endpoint. In this trial, the loading dose and maintenance dose of leflunomide was based on three weight categories: <20 kg, 20 to 40kg, and >40 kg. The response rate to leflunomide in pediatric patients ≤40 kg was less robust than in pediatric patients >40 kg suggesting suboptimal dosing in smaller weight pediatric patients, as studied, resulting in less than efficacious plasma concentrations, despite reduced clearance of M1.
How Supplied
Storage
There is limited information regarding Leflunomide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Leflunomide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Leflunomide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Leflunomide in the drug label.
Precautions with Alcohol
- Alcohol-Leflunomide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Leflunomide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Leflunomide |Label Name=Leflunomide11.png
}}
{{#subobject:
|Label Page=Leflunomide |Label Name=Leflunomide11.png
}}