Ivacaftor
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ivacaftor is a cystic fibrosis transmembrane conductance regulator that is FDA approved for the treatment of cystic fibrosis. Common adverse reactions include headache, oropharyngeal pain, upper respiratory tract infection, nasal congestion, abdominal pain, nasopharyngitis, diarrhea, rash, nausea, and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cystic Fibrosis
- Dosage: One 150 mg tablet taken orally every 12 hours (300 mg total daily dose) with fat-containing food. Examples of appropriate fat-containing foods include eggs, butter, peanut butter, cheese pizza,
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivacaftor in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ivacaftor in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Cystic Fibrosis
- Dosage: One 150 mg tablet taken orally every 12 hours (300 mg total daily dose) with fat-containing food. Examples of appropriate fat-containing foods include eggs, butter, peanut butter, cheese pizza,
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivacaftor in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ivacaftor in pediatric patients.
Contraindications
None
Warnings
Transaminase (ALT or AST) Elevations
Elevated transaminases have been reported in patients with CF receiving KALYDECO. It is recommended that ALT and AST be assessed prior to initiating KALYDECO, every 3 months during the first year of treatment, and annually thereafter. Patients who develop increased transaminase levels should be closely monitored until the abnormalities resolve. Dosing should be interrupted in patients with ALT or AST of greater than 5 times the upper limit of normal (ULN). Following resolution of transaminase elevations, consider the benefits and risks of resuming KALYDECO dosing.
Concomitant Use with CYP3A Inducers
Use of KALYDECO with strong CYP3A inducers, such as rifampin, substantially decreases the exposure of ivacaftor, which may reduce the therapeutic effectiveness of KALYDECO. Therefore, co-administration of KALYDECO with strong CYP3A inducers (e.g., rifampin, St. John's Wort) is not recommended.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. The overall safety profile of KALYDECO is based on pooled data from three placebo-controlled clinical trials conducted in 353 patients with CF who had a G551D mutation in the CFTR gene (Trials 1 and 2) or were homozygous for the F508del mutation (Trial 3). In addition, an 8-week crossover design trial (Trial 4) involving 39 patients with a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene was conducted. Patients treated with KALYDECO in these trials were between the ages of 6 and 57 years.
Of the 353 patients included in the pooled analyses of patients with CF who had either a G551D mutation or were homozygous for the F508del mutation in the CFTR gene, 50% of patients were female and 97% were Caucasian; 221 received KALYDECO and 132 received placebo from 16 to 48 weeks. The proportion of patients who prematurely discontinued study drug due to adverse reactions was 2% for KALYDECO-treated patients and 5% for placebo-treated patients. Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in KALYDECO-treated patients included abdominal pain, increased hepatic enzymes, and hypoglycemia.
The most common adverse reactions in the 221 patients treated with KALYDECO were headache (17%), upper respiratory tract infection (16%), nasal congestion (16%), nausea]] (10%), rash (10%), rhinitis (6%), dizziness (5%), arthralgia (5%), and bacteria in sputum (5%).
The incidence of adverse reactions below is based upon two double-blind, placebo-controlled, 48-week clinical trials (Trials 1 and 2) in a total of 213 patients with CF ages 6 to 53 who have a G551D mutation in the CFTR gene and who were treated with KALYDECO 150 mg orally or placebo twice daily. Table 1 shows adverse reactions occurring in ≥8% of KALYDECO-treated patients with CF who have a G551D mutation in the CFTR gene that also occurred at a higher rate than in the placebo-treated patients in the two double-blind, placebo-controlled trials.
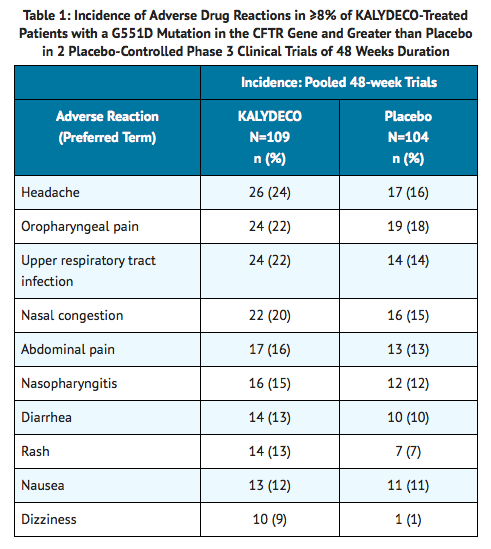
Adverse reactions in the 48-week clinical trials that occurred in the KALYDECO group at a frequency of 4 to 7% where rates exceeded that in the placebo group include:
- Infections and infestations: rhinitis
- Investigations: aspartate aminotransferase increased, bacteria in sputum, blood glucose increased, hepatic enzyme increased
- Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal chest pain, myalgia
- Nervous system disorders: sinus headache
- Respiratory, thoracic and mediastinal disorders: pharyngeal erythema, pleuritic pain, sinus congestion, wheezing.
- Skin and subcutaneous tissue disorders: acne
- Laboratory abnormalities:
- Transaminase Elevations: During 48-week placebo-controlled clinical studies, the incidence of maximum transaminase (ALT or AST) >8, >5 or >3 × ULN was 2%, 3% and 6% in KALYDECO-treated patients and 2%, 2% and 8% in placebo-treated patients, respectively. Two patients (2%) on placebo and 1 patient (0.5 %) on KALYDECO permanently discontinued treatment for elevated transaminases, all >8 × ULN. Two patients treated with KALYDECO were reported to have serious adverse reactions of elevated liver transaminases compared to none on placebo.
Postmarketing Experience
There is limited information regarding Ivacaftor Postmarketing Experience in the drug label.
Drug Interactions
Inhibitors of CYP3A
Ivacaftor is a sensitive CYP3A substrate. Co-administration with ketoconazole, a strong CYP3A inhibitor, significantly increased ivacaftor exposure measured as area under the curve (AUC) by 8.5-fold. Based on simulations of these results, a reduction of the KALYDECO dose to 150 mg twice a week is recommended for co-administration with strong CYP3A inhibitors, such as ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin, and clarithromycin. Co-administration with fluconazole, a moderate inhibitor of CYP3A, increased ivacaftor exposure by 3-fold. Therefore, a reduction of the KALYDECO dose to 150 mg once daily is recommended for patients taking concomitant moderate CYP3A inhibitors, such as fluconazole and erythromycin.
Co-administration of KALYDECO with grapefruit juice, which contains one or more components that moderately inhibit CYP3A, may increase exposure of ivacaftor. Therefore, food containing grapefruit or Seville oranges should be avoided during treatment with KALYDECO [see CLINICAL PHARMACOLOGY (12.3)].
Inducers of CYP3A
Co-administration with rifampin, a strong CYP3A inducer, significantly decreased ivacaftor exposure (AUC) by approximately 9-fold. Therefore, co-administration with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's Wort is not recommended.
Potential for ivacaftor to affect other drugs
CYP3A and/or P-gp Substrates
Ivacaftor and its M1 metabolite have the potential to inhibit CYP3A and P-gp. Co-administration with midazolam, a sensitive CYP3A substrate, increased midazolam exposure 1.5-fold, consistent with weak inhibition of CYP3A by ivacaftor. Co-administration with digoxin, a sensitive P-gp substrate, increased digoxin exposure by 1.3-fold, consistent with weak inhibition of P-gp by ivacaftor. Administration of KALYDECO may increase systemic exposure of drugs that are substrates of CYP3A and/or P-gp, which may increase or prolong their therapeutic effect and adverse events. Therefore, caution and appropriate monitoring are recommended when co-administering KALYDECO with CYP3A and/or P-gp substrates, such as digoxin, cyclosporine, and tacrolimus.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
There are no adequate and well-controlled studies of KALYDECO in pregnant women. Ivacaftor was not teratogenic in rats at approximately 6 times the maximum recommended human dose (MRHD) (based on summed AUCs for ivacaftor and its metabolites at a maternal dose of 200 mg/kg/day). Ivacaftor was not teratogenic in rabbits at approximately 12 times the MRHD (on an ivacaftor AUC basis at a maternal dose of 100 mg/kg/day, respectively). Placental transfer of ivacaftor was observed in pregnant rats and rabbits. Because animal reproduction studies are not always predictive of human response, KALYDECO should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ivacaftor in women who are pregnant.
Labor and Delivery
Ivacaftor is excreted into the milk of lactating female rats. Excretion of ivacaftor into human milk is probable. There are no human studies that have investigated the effects of ivacaftor on breast-fed infants. Caution should be exercised when KALYDECO is administered to a nursing woman.
Nursing Mothers
There is no FDA guidance on the use of Ivacaftor in women who are nursing.
Pediatric Use
he safety and efficacy of KALYDECO in patients 6 to 17 years of age with CF who have a G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene has been demonstrated [see ADVERSE REACTIONS (6) and CLINICAL STUDIES (14)].
The safety and efficacy of KALYDECO in patients with CF younger than age 6 years have not been established.
Geriatic Use
CF is largely a disease of children and young adults. Clinical trials of KALYDECO did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Ivacaftor with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ivacaftor with respect to specific racial populations.
Renal Impairment
KALYDECO has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is necessary for patients with mild to moderate renal impairment; however, caution is recommended while using KALYDECO in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min) or end-stage renal disease.
Hepatic Impairment
No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A reduced dose of 150 mg once daily is recommended in patients with moderate hepatic impairment (Child-Pugh Class B). Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C) but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a dose of 150 mg once daily or less frequently in patients with severe hepatic impairment after weighing the risks and benefits of treatment
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ivacaftor in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ivacaftor in patients who are immunocompromised.
Patients with CF who are Homozygous for the F508del Mutation in the CFTR Gene
Efficacy results from a double-blind, placebo-controlled trial in patients with CF who are homozygous for the F508del mutation in the CFTR gene showed no statistically significant difference in forced expiratory volume exhaled in one second (FEV1) over 16 weeks of KALYDECO treatment compared to placebo [see CLINICAL STUDIES (14.3)]. Therefore, KALYDECO should not be used in patients homozygous for the F508del mutation in the CFTR gene.
Administration and Monitoring
Administration
There is limited information regarding Ivacaftor Administration in the drug label.
Monitoring
There is limited information regarding Ivacaftor Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ivacaftor and IV administrations.
Overdosage
There is limited information regarding Ivacaftor overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Ivacaftor Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Ivacaftor Mechanism of Action in the drug label.
Structure
There is limited information regarding Ivacaftor Structure in the drug label.
Pharmacodynamics
There is limited information regarding Ivacaftor Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Ivacaftor Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Ivacaftor Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ivacaftor Clinical Studies in the drug label.
How Supplied
There is limited information regarding Ivacaftor How Supplied in the drug label.
Storage
There is limited information regarding Ivacaftor Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ivacaftor |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ivacaftor |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ivacaftor Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ivacaftor interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ivacaftor Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ivacaftor Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.