Isosorbide dinitrate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Isosorbide dinitrate is an anti-anginal nitrate that is FDA approved for the {{{indicationType}}} of angina pectoris due to coronary artery disease. Common adverse reactions include hypotension, lightheadedness, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Angina Pectoris
- Isosorbide dinitrate are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of immediate-release oral isosorbide dinitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
- A daily dose-free interval of at least 14 hours is advisable to minimize tolerance. The optimal interval will vary with the individual patient, dose and regimen.
- Multiple-dose studies with ISDN and other nitrates have shown that maintenance of continuous 24-hour plasma levels results in refractory tolerance. Every dosing regimen for Isordil Titradose tablets must provide a daily dose-free interval to minimize the development of this tolerance. With immediate-release ISDN, it appears that one daily dose-free interval must be at least 14 hours long.
- The effects of the second and later doses have been smaller and shorter-lasting than the effects of the first.
- Large controlled studies with other nitrates suggest that no dosing regimen with Isordil Titradose tablets should be expected to provide more than about 12 hours of continuous anti-anginal efficacy per day.
- Dosing Information
- Initial Dosage
- 5–20 mg PO bid–tid
- Maintenance Dosage
- 10–40 mg PO bid–tid
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Ischemic Symptoms of Unstable Angina
- Developed by: ACCF/AHA
- Class of Recommendation: Class I
- Strength of Evidence: Level C
- Dosing Information
- 5–80 mg, 2 or 3 times daily[1]
Non–Guideline-Supported Use
Cardiac Syndrome X
- Dosing Information
- 5 mg sublingual[2]
Congestive Heart Failure
- Dosing Information
- Hydralazine-isosorbide dinitrate (H-ID) 37.5/20 mg 3 times daily, titrated to 75/40 mg 3 times daily[3]
- Addition of isosorbide dinitrate 40 mg four times daily and hydralazine 300 mg daily to a digoxin/diuretic combination[4][5]
Myocardial Infarction
- Dosing Information
- Initial dose was 2 mg/hour, titrated every 10 minutes until systolic blood pressure decreased 10% or a maximum infusion rate of 10 mg/hour.[6]
Pulmonary Edema
- Dosing Information
- Repeated boluses of IV isosorbide dinitrate 4 mg every 4 min, until oxygen saturation increased above 96% or systolic blood pressure fell below 110 mm Hg or 30% below baseline.[7]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isosorbide dinitrate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Isosorbide dinitrate in pediatric patients.
Contraindications
- Allergic reactions to organic nitrates are extremely rare, but they do occur. Isordil is contraindicated in patients who are allergic to isosorbide dinitrate or any of its other ingredients.
Warnings
- Amplification of the vasodilatory effects of Isordil by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the the extremities and with central volume expansion.
- The benefits of immediate-release oral isosorbide dinitrate in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use isosorbide dinitrate in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia. Because the effects of oral isosorbide dinitrate are so difficult to terminate rapidly, this formulation is not recommended in these settings.
Precautions
- Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide dinitrate. This drug should therefore be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide dinitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
- Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
- As tolerance to isosorbide dinitrate develops, the effect of sublingual nitroglycerin on exercise tolerance, although still observable, is somewhat blunted.
- Some clinical trials in angina patients have provided nitroglycerin for about 12 continuous hours of every 24-hour day. During the daily dose-free interval in some of these trials, anginal attacks have been more easily provoked than before treatment, and patients have demonstrated hemodynamic rebound and decreased exercise tolerance. The importance of these observations to the routine, clinical use of immediate-release oral isosorbide dinitrate is not known.
- In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Isosorbide dinitrate in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Isosorbide dinitrate in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Isosorbide dinitrate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Isosorbide dinitrate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Isosorbide dinitrate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Isosorbide dinitrate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Isosorbide dinitrate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Isosorbide dinitrate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Isosorbide dinitrate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Isosorbide dinitrate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Isosorbide dinitrate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Isosorbide dinitrate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Isosorbide dinitrate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Isosorbide dinitrate in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Isosorbide dinitrate in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Isosorbide dinitrate in the drug label.
Pharmacology
| |
| |
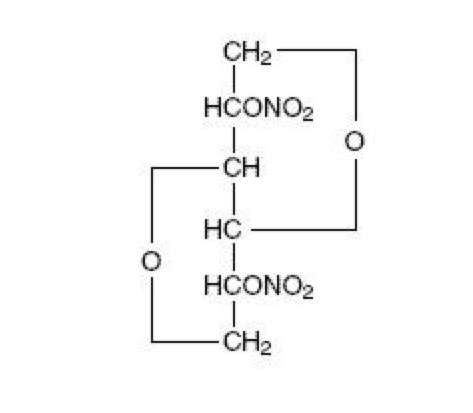
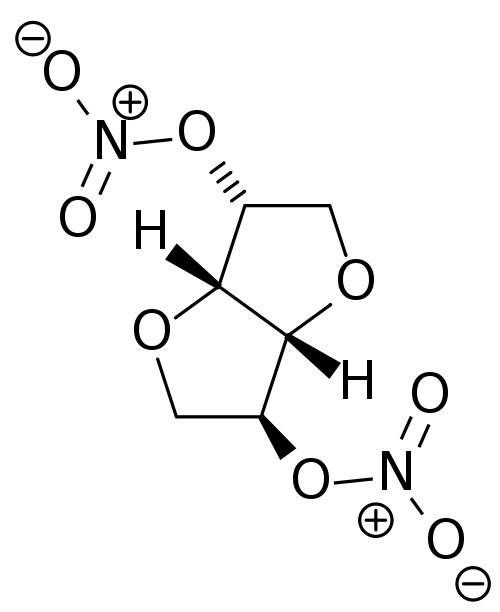
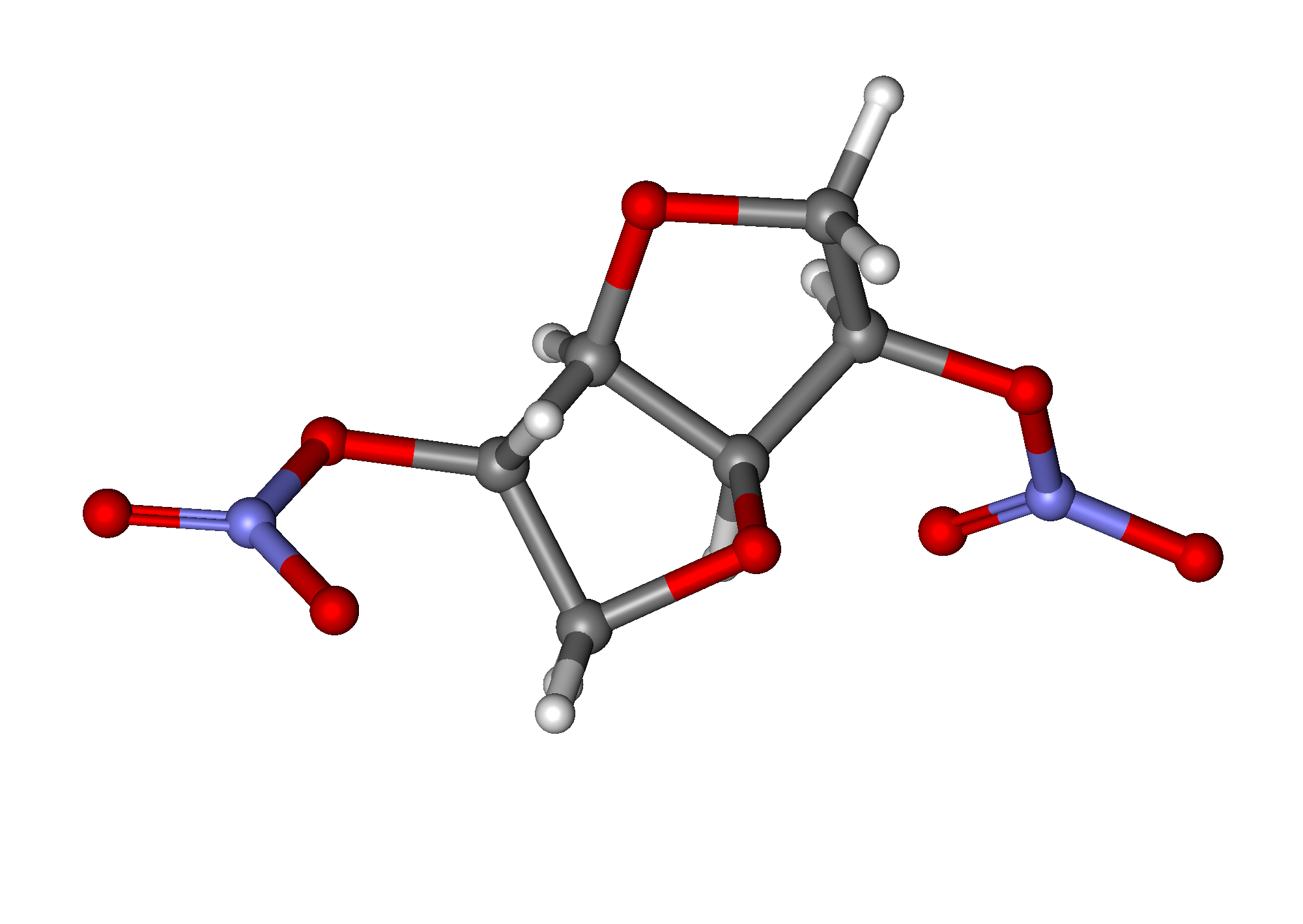
Isosorbide dinitrate
| |
| Systematic (IUPAC) name | |
| 1,4:3,6-dianhydro-2,5-di-O-nitro-D-glucitol | |
| Identifiers | |
| CAS number | |
| ATC code | C01 C05AE02 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 236.136 g/mol |
| SMILES | & |
| Synonyms | (3R,3aS,6S,6aS)-hexahydrofuro[3,2-b]furan-3,6-diyl dinitrate |
| Pharmacokinetic data | |
| Bioavailability | 10–90%, average 25% |
| Metabolism | Hepatic |
| Half life | 1 hour |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Isosorbide dinitrate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Isosorbide dinitrate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Isosorbide dinitrate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Isosorbide dinitrate in the drug label.
Condition1
- Description
How Supplied
- Isordil® (isosorbide dinitrate) Oral Titradose™ Tablets are available as follows:
- 5 mg, round, pink tablets imprinted "BPI 152" on one side and deeply scored on reverse side
- NDC 64455-152-01, bottles of 100.
- 40 mg, round, light green tablets imprinted "BPI 192" on one side and deeply scored on reverse side:
- NDC 64455-192-01, bottles of 100.
- Store at controlled room temperature, 25°C (77°F); excursions permitted to 15 -30° (59-86°F)
- Protect from light.
- Keep bottles tightly closed.
- Dispense in a light-resistant, tight container.
Storage
There is limited information regarding Isosorbide dinitrate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Isosorbide dinitrate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Isosorbide dinitrate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be told that the anti-anginal efficacy of isosorbide dinitrate is strongly related to its dosing regimen, so the prescribed schedule of dosing should be followed carefully. In particular, daily headaches sometimes accompany treatment with isosorbide dinitrate. In patients who get these headaches, the headaches are a marker of the activity of the drug. Patients should resist the temptation to avoid headaches by altering the schedule of their treatment with isosorbide dinitrate, since loss of headache may be associated with simultaneous loss of anti-anginal efficacy. Aspirin and/or acetaminophen, on the other hand, often successfully relieve isosorbide dinitrate-induced headaches with no deleterious effect on isosorbide dinitrate's anti-anginal efficacy.
- Treatment with isosorbide dinitrate may be associated with lightheadedness on standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
Precautions with Alcohol
- Alcohol-Isosorbide dinitrate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Isordil®[8]
Look-Alike Drug Names
- Isordil® — Plendil®[9]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Anderson, Jeffrey L. (2007-08-14). "ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine". Journal of the American College of Cardiology. 50 (7): –1-e157. doi:10.1016/j.jacc.2007.02.013. ISSN 1558-3597. PMID 17692738. Unknown parameter
|coauthors=ignored (help) - ↑ Lanza, G. A. (1994-12). "Acute effects of nitrates on exercise testing in patients with syndrome X. Clinical and pathophysiological implications". Circulation. 90 (6): 2695–2700. ISSN 0009-7322. PMID 7994810. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Taylor, Anne L. (2004-11-11). "Combination of isosorbide dinitrate and hydralazine in blacks with heart failure". The New England Journal of Medicine. 351 (20): 2049–2057. doi:10.1056/NEJMoa042934. ISSN 1533-4406. PMID 15533851. Unknown parameter
|coauthors=ignored (help) - ↑ Cohn, J. N. (1986-06-12). "Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study". The New England Journal of Medicine. 314 (24): 1547–1552. doi:10.1056/NEJM198606123142404. ISSN 0028-4793. PMID 3520315. Unknown parameter
|coauthors=ignored (help) - ↑ Cohn, J. N. (1993-10). "Efficacy of vasodilators in the treatment of heart failure". Journal of the American College of Cardiology. 22 (4 Suppl A): 135–138A. ISSN 0735-1097. PMID 8376683. Check date values in:
|date=(help) - ↑ Hildebrandt, P. (1992-11). "Reduced infarct size in nonreperfused myocardial infarction by combined infusion of isosorbide dinitrate and streptokinase". American Heart Journal. 124 (5): 1139–1144. ISSN 0002-8703. PMID 1442478. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Sharon, A. (2000-09). "High-dose intravenous isosorbide-dinitrate is safer and better than Bi-PAP ventilation combined with conventional treatment for severe pulmonary edema". Journal of the American College of Cardiology. 36 (3): 832–837. ISSN 0735-1097. PMID 10987607. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "ISORDIL TITRADOSE (isosorbide dinitrate) tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Isosorbide dinitrate |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Isosorbide dinitrate |Label Name=Isosorbide dinitrate02.png
}}
{{#subobject:
|Label Page=Isosorbide dinitrate |Label Name=Isosorbide dinitrate03.png
}}
{{#subobject:
|Label Page=Isosorbide dinitrate |Label Name=Isosorbide dinitrate04.png
}}