Intravenous leiomyomatosis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
==Historical Perspective== | ==Historical Perspective== | ||
*first described in 1896 German Pathologist Birch-Hirschfeld | *first described in 1896 German Pathologist Birch-Hirschfeld <nowiki>PMID 11904348</nowiki> | ||
*first reported case of leiomyo-matosis of pelvic origin with intravascular extension to cardiac cavities was described in 1907 in Germany by Dürck and Hörmann. <ref><nowiki>https://www.ncbi.nlm.nih.gov/pubmed/23563052</nowiki></ref> | *first reported case of leiomyo-matosis of pelvic origin with intravascular extension to cardiac cavities was described in 1907 in Germany by Dürck and Hörmann. <ref><nowiki>https://www.ncbi.nlm.nih.gov/pubmed/23563052</nowiki></ref> | ||
| Line 49: | Line 49: | ||
===Race=== | ===Race=== | ||
*There is no racial predilection | *There is no racial predilection for intravenous leiomyomatosis. | ||
==Risk Factors== | ==Risk Factors== | ||
| Line 67: | Line 67: | ||
== Diagnosis == | == Diagnosis == | ||
===Diagnostic Criteria=== | ===Diagnostic Criteria=== | ||
* | *There is no specific diagnostic Criteria for intravenous leiomyomatosis. Early diagnosis may be difficult because patients may be asymptomatic. How ever the diagnosis of intravenous leiomyomatosis is made when following findings are seen: | ||
:* | |||
:* | :*benign tumors arising from smooth muscle of the uterus PMID: 24027407 | ||
:* | :*proliferating smooth muscle cells PMID: 20492407 | ||
:* | :*intravenous plug is mainly smooth muscle in origin PMID: 11904348 | ||
:*tumor cells invaginating the vascular tree with no evidence of atypi PMID: 24027407 | |||
=== Symptoms === | === Symptoms === | ||
| Line 118: | Line 119: | ||
=== Laboratory Findings === | === Laboratory Findings === | ||
*There are no specific laboratory findings associated with [ | *There are no specific laboratory findings associated with [intravenous leiomyomatosis]. | ||
*A [positive/negative] [test name] is diagnostic of [disease name]. | *A [positive/negative] [test name] is diagnostic of [disease name]. | ||
| Line 130: | Line 131: | ||
*On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3]. | *On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3]. | ||
*[Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3]. | *[Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3]. | ||
== Treatment == | == Treatment == | ||
Revision as of 18:12, 11 August 2019
1Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Ammu Susheela, M.D. [3]
Synonyms and keywords: Nesidioblastoma, IVLM
Overview
Intravenous leiomyomatosis is characterized by the extension into venous channels of histologically benign smooth muscle tumor arising from either the wall of a vessel or from a uterine leiomyoma. The etiology of intravenous leiomyomatosis is unclear. Intravenous leiomyomatosis must be differentiated from other diseases such as renal malignancies and sarcoma. The median age is 45 years, with patients ranging from 26 to 70 years old. Only females may develop intravenous leiomyomatosis. Common complications of intravenous leiomyomatosis include embolization, recurrence of the tumor, and metastasis. Surgery is the mainstay of therapy for intravenous leiomyomatosis. It can grow in to lymphatics /veins.[1]
Historical Perspective
- first described in 1896 German Pathologist Birch-Hirschfeld PMID 11904348
- first reported case of leiomyo-matosis of pelvic origin with intravascular extension to cardiac cavities was described in 1907 in Germany by Dürck and Hörmann. [2]
Pathophysiology
- Intravenous leiomyomatosis is characterized by the extension into venous channels of histologically benign smooth muscle tumor arising from either the wall of a vessel or from a uterine leiomyoma.[3]
- Approximately 40% of leiomyomata have cytogenetic abnormalities.
- They are benign tumors of uterus that extend to veins system but do not invade the surrounding tissues [4]
- They contains receptors to Estrogen and progesterone and hence response to these hormones [5]
- It is also referred as quasi-malignant behavior due to its speedy spreading behavior. PMID 11904348
- Patients are exclusively female, and the majority are white, premenopausal, and parous.PMID 12649198
- Intravenous leiomyomatosis should be considered in young women with cardiac symptoms who have a right atrial mass as well as a pelvic mass or who have previously undergone hysterectomy for leiomyoma uterus with intravenous involvement.(PMID 12508249)
Causes
- The etiology of intravenous leiomyomatosis is unclear.[6]
- Only two cytogenetic reports in IVL and both exhibited a karyotype with a der(14)t(12;14)(q15;q24) and two normal copies of chromosome 12 which has close association to uterine leiomyoma genetics
Differentiating Intravenous Leiomyomatosis from other Diseases
- Intravenous leiomyomatosis must be differentiated from other diseases such as:
- Renal malignancies
- Sarcoma
- Thrombosis of the intravenous catheter
Epidemiology and Demographics
- The median age is 45 years, with patients ranging from 26 to 70 years old.
- Female are exclusively affected with intravenous leiomyomatosis.
- The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide.
- In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location].
Age
- Patients affected are of reproductive age group
- Age from 28 to 76 years (median, 42 years) (DOI: S0368-2315(04)96638-0)
Gender
- It is diagnosis only of female
Race
- There is no racial predilection for intravenous leiomyomatosis.
Risk Factors
- Common risk factors in the development of ntravenous leiomyomatosis are age, cytogenetics , and prior history,
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%].
- Common complications of intravenous leiomyomatosis include embolization, recurrence of the tumor, and metastasis.
- The tumor is slow growing, and the prognosis is favorable.
Diagnosis
Diagnostic Criteria
- There is no specific diagnostic Criteria for intravenous leiomyomatosis. Early diagnosis may be difficult because patients may be asymptomatic. How ever the diagnosis of intravenous leiomyomatosis is made when following findings are seen:
- benign tumors arising from smooth muscle of the uterus PMID: 24027407
- proliferating smooth muscle cells PMID: 20492407
- intravenous plug is mainly smooth muscle in origin PMID: 11904348
- tumor cells invaginating the vascular tree with no evidence of atypi PMID: 24027407
Symptoms
Patients may be asymptomatic or have symptoms of uterine leiomyomas, syncopal episodes, and dyspnea on exertion.
- Symptoms of intravenous Leiomyomatosis may include the following:
- Dyspnoea
- Syncope
- Oedema of the lower extremities
- Palpitation
- Fatigue
- Ascites
- Jugular vein distension
- Chest pain
- Abdominal pain
- Asymptomatic
Physical Examination
- Patients with intravenous Leiomyomatosis usually depends on location and extension which may include as :
- Iliac vein
- Ovarian vein
- Renal vein
- Both iliac and ovarian veins
- Gonadal vein
- Hypogastric vein
- Uterine vein
- Hypohepatic or hepatic vein
- Pelvic vein
- Physical examination may be remarkable for:
- [finding 1]
- [finding 2]
- [finding 3]
- [finding 4]
- [finding 5]
- [finding 6]
Laboratory Findings
- There are no specific laboratory findings associated with [intravenous leiomyomatosis].
- A [positive/negative] [test name] is diagnostic of [disease name].
- An [elevated/reduced] concentration of [serum/blood/urinary/CSF/other] [lab test] is diagnostic of [disease name].
- Other laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
Imaging Findings
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action 1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
References
- ↑ Mariyappa N, Manikyam UK, Krishnamurthy D, Preeti K, Agarwal Y, Prakar U (2012). "Intravenous leiomyomatosis". Niger J Surg. 18 (2): 105–6. doi:10.4103/1117-6806.103122. PMC 3762011. PMID 24027407.
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/23563052
- ↑ Mariyappa N, Manikyam UK, Krishnamurthy D, Preeti K, Agarwal Y, Prakar U (2012). "Intravenous leiomyomatosis". Niger J Surg. 18 (2): 105–6. doi:10.4103/1117-6806.103122. PMC 3762011. PMID 24027407.
- ↑ Dal Cin P, Quade BJ, Neskey DM, Kleinman MS, Weremowicz S, Morton CC (2003). "Intravenous leiomyomatosis is characterized by a der(14)t(12;14)(q15;q24)". Genes Chromosomes Cancer. 36 (2): 205–6. doi:10.1002/gcc.10159. PMID 12508249.
- ↑ Dal Cin P, Quade BJ, Neskey DM, Kleinman MS, Weremowicz S, Morton CC (2003). "Intravenous leiomyomatosis is characterized by a der(14)t(12;14)(q15;q24)". Genes Chromosomes Cancer. 36 (2): 205–6. doi:10.1002/gcc.10159. PMID 12508249.
- ↑ "Leiomyomas beyond the Uterus: Unusual Locations, Rare Manifestations1". line feed character in
|title=at position 18 (help)
Images
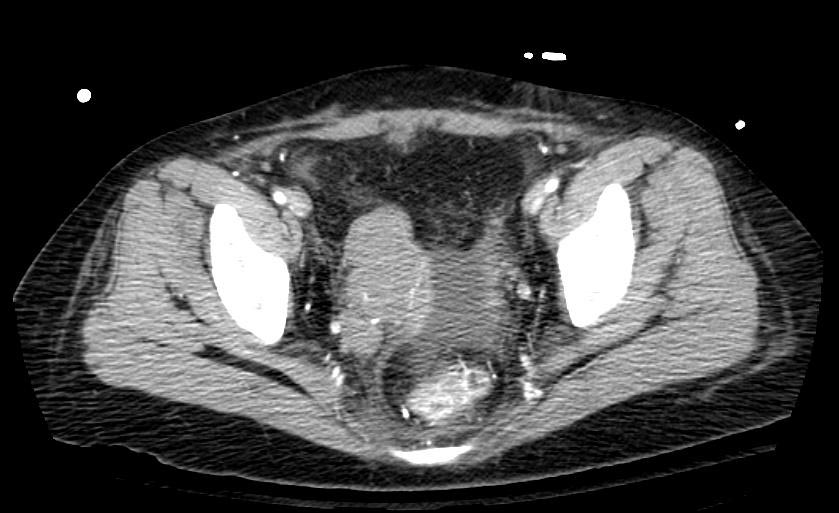
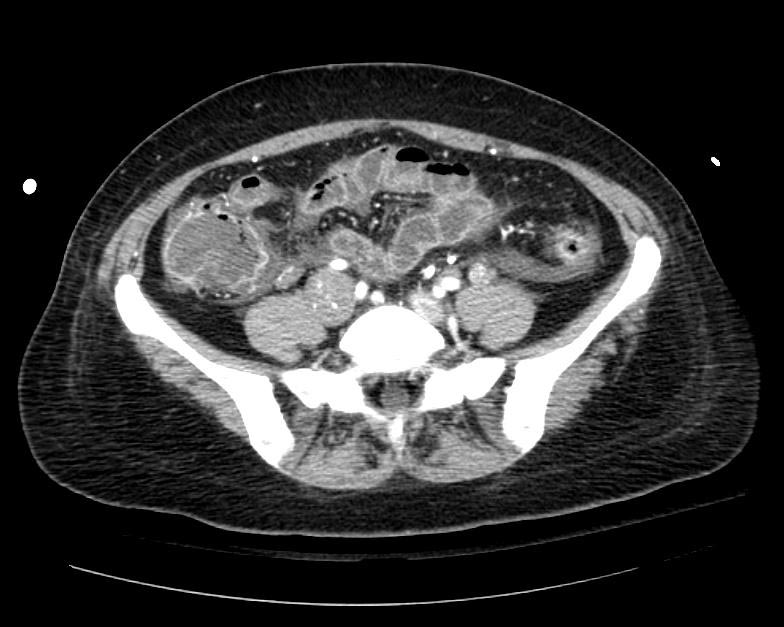
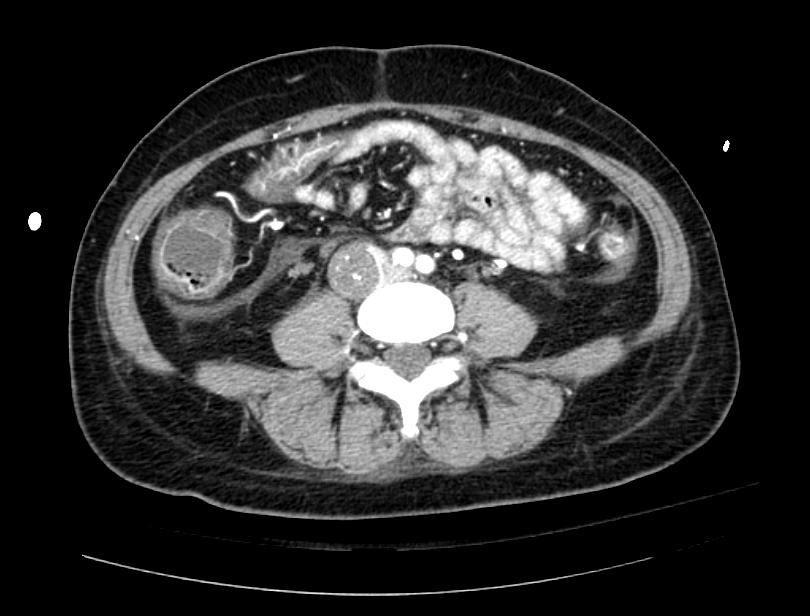
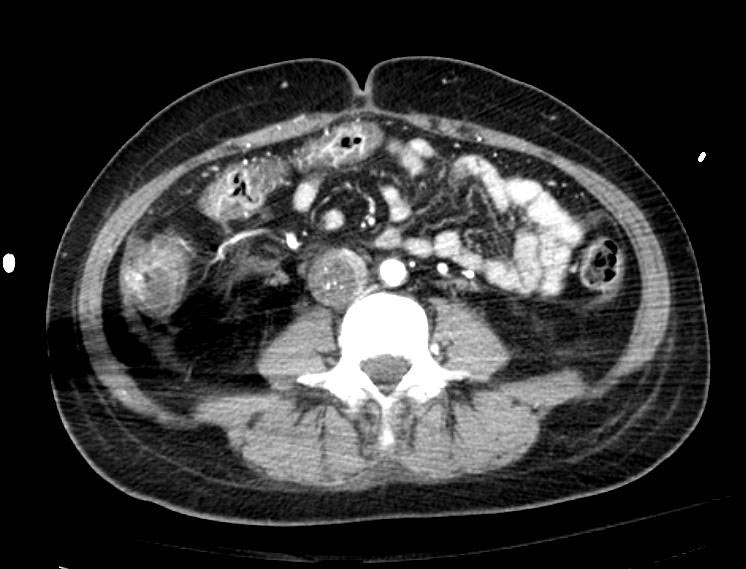
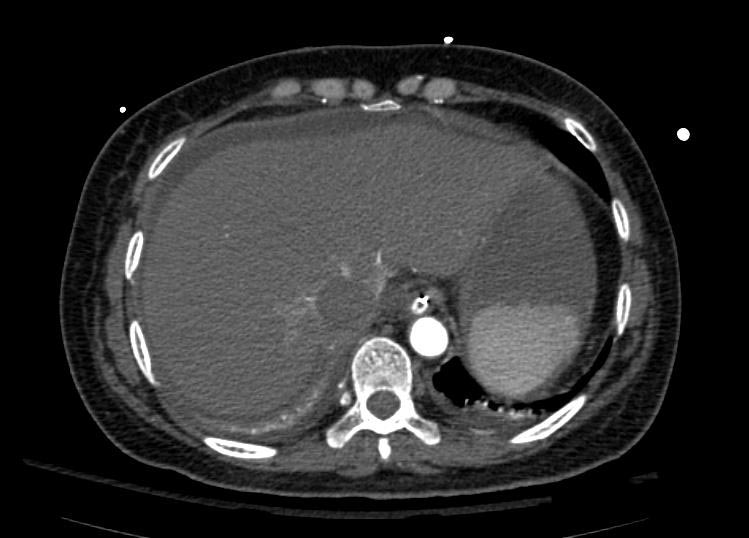
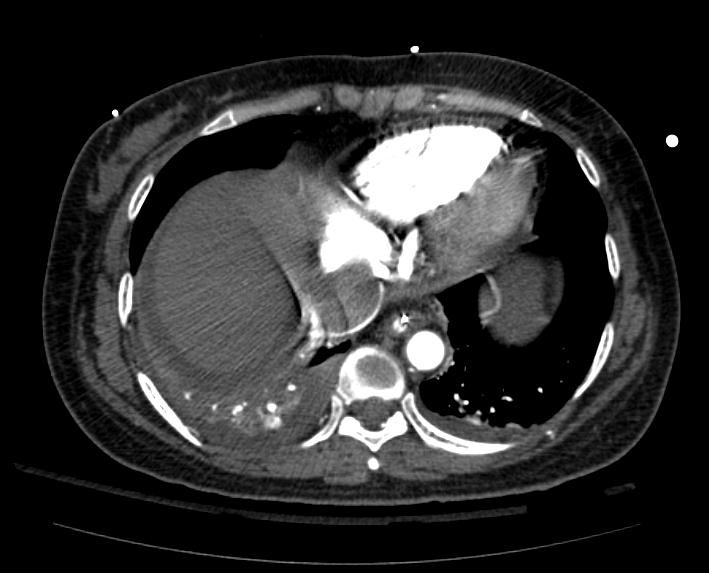
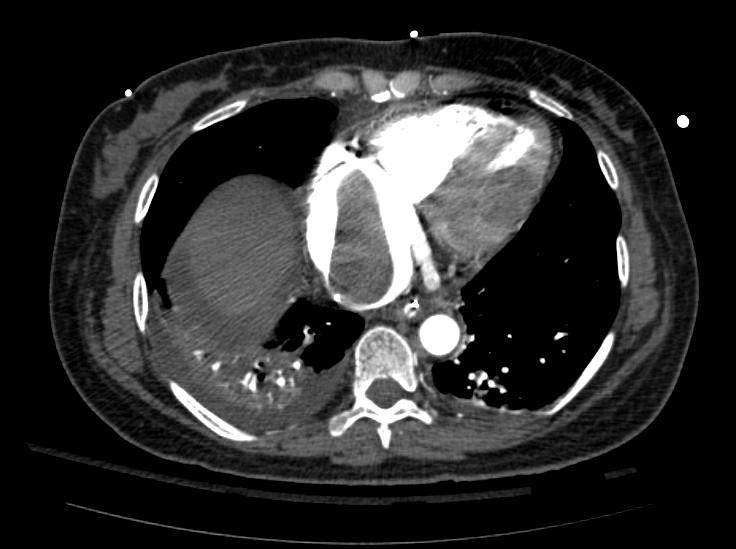
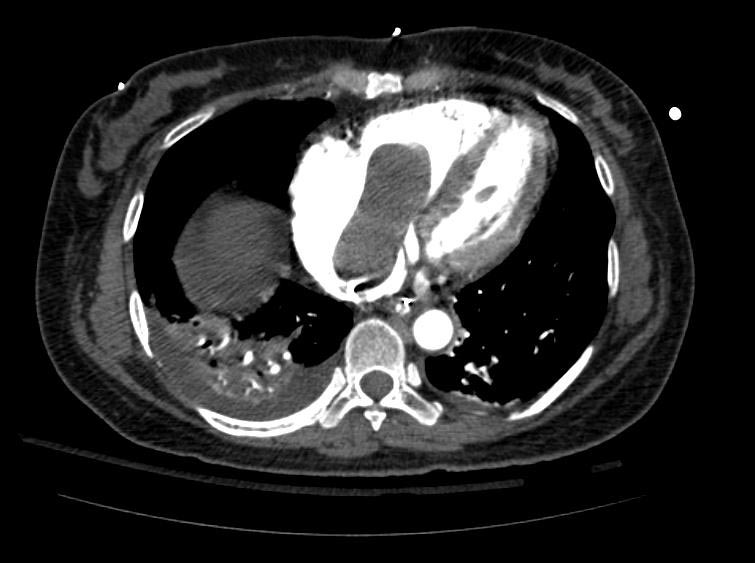
Example #1
The patient presented with S.O.B. one year after hysterectomy for a leiomyomatous uterus.
Treatment
- Surgery is the mainstay of therapy for intravenous leiomyomatosis.
Related Chapters
- Uterine leiomyoma
- Benign metastasizing leiomyoma