Indigotindisulfonate: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Indigotindisulfonate": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (4 intermediate revisions by one other user not shown) | |||
| Line 3: | Line 3: | ||
|genericName=Indigotindisulfonate sodium | |genericName=Indigotindisulfonate sodium | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=Diagnostic Agent | |drugClass=[[Diagnosis|Diagnostic Agent]] | ||
|indicationType=diagnosis | |indicationType=diagnosis | ||
|indication=ureteral orifices during cystoscopy and ureteral catheterization | |indication=[[Ureter|ureteral orifices]] during [[cystoscopy]] and [[Catheter|ureteral catheterization]] | ||
|adverseReactions=allergic reaction | |adverseReactions=[[allergic reaction]] | ||
| Line 20: | Line 20: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=====Indications==== | |fdaLIADAdult=====Indications==== | ||
Originally employed as a kidney function test, the chief application of Indigo Carmine at present is localizing ureteral orifices during cystoscopy and ureteral catheterization. | * Originally employed as a [[kidney function test]], the chief application of Indigo Carmine at present is localizing [[Ureter|ureteral orifices]] during [[cystoscopy]] and [[Catheter|ureteral catheterization]]. | ||
====Dosage==== | ====Dosage==== | ||
Indigo Carmine solution is injected either by the intravenous or intramuscular route, and its appearance at the ureteral orifices is watched with the cystoscope in place. The intravenous method is preferred because a 5 mL injection is sufficient. A lesser dosage in infants, children and underweight patients will prevent skin coloration. | * Indigo Carmine solution is injected either by the [[intravenous]] or [[intramuscular]] route, and its appearance at the [[Ureter|ureteral orifices]] is watched with the [[cystoscope]] in place. The [[intravenous]] method is preferred because a 5 mL injection is sufficient. A lesser dosage in infants, children and underweight patients will prevent skin coloration. | ||
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. | * Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 41: | Line 41: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=* Indigo Carmine is contraindicated in patients who have previously experienced an adverse reaction following its use. | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=* | |warnings=* An occasional idiosyncratic [[drug reaction]] may occur. A mild pressor effect may be encountered in some patients. | ||
* Since precipitation of indigotindisulfonate sodium may occur, Indigo Carmine Solution must not be diluted prior to injection or injected with infusion assemblies which were used with other solutions. | |||
====Precautions==== | ====Precautions==== | ||
* | * Indigo Carmine should be stored in the dark, away from direct light, preferably in the original package. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing= | |postmarketing=[[allergic reaction]] | ||
|drugInteractions=<!--Use in Specific Populations--> | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=* Animal Reproduction studies have not been conducted with indigotindisulfonate sodium injection. It is also not known whether indigotindisulfonate sodium injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indigotindisulfonate sodium injection should be given to a pregnant woman only if clearly needed. | |||
|drugInteractions= | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA=* | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Indigo Carmine is administered to a nursing woman. | ||
|useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
| Line 195: | Line 77: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* [[Intravenous]] | ||
* [[Intramuscular]] | |||
* | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 206: | Line 86: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=* There are no data available describing the signs, symptoms or laboratory findings accompanying overdosage. | ||
* | |||
* No discernible symptoms of toxicity have been observed in mice with an intravenous dose of 200 mg/kg. After intravenous administration the LD80 was established at 300 mg/kg in mice. | |||
====DRUG ABUSE AND DEPENDENCE==== | |||
* Indigo Carmine is not a controlled substance listed in any of the Drug Enforcement Administration Schedules. Its use is not known to lead to dependence or abuse. | |||
=== | |||
|drugBox=<!--Mechanism of Action--> | |drugBox=<!--Mechanism of Action--> | ||
|mechAction=* | |mechAction=* | ||
<!--Structure--> | <!--Structure--> | ||
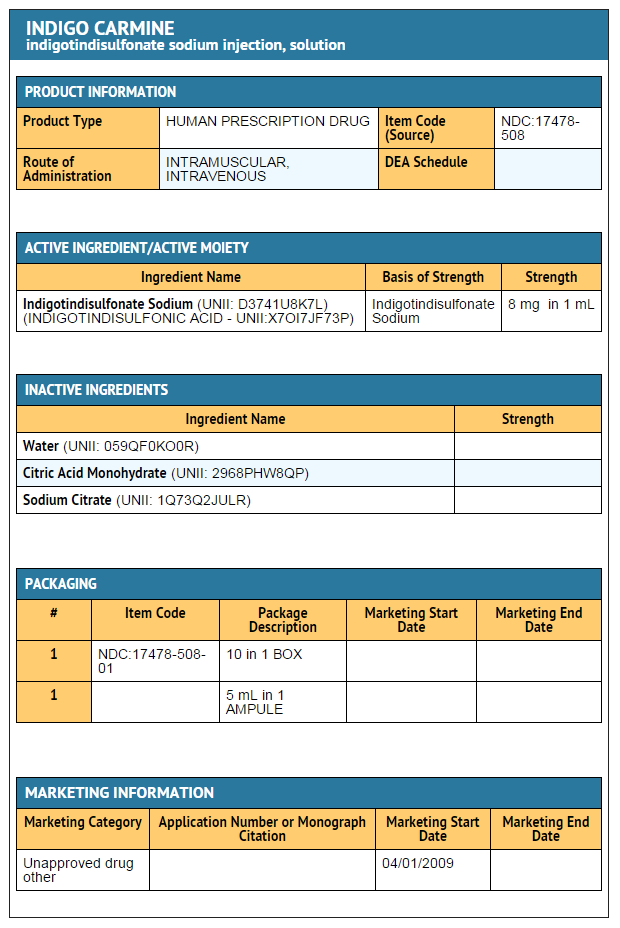
|structure=* | |structure=* Each mL contains: | ||
* Active: Indigotindisulfonate Sodium 8 mg Inactives: Water for injection, q.s. pH adjusted when necessary with Citric Acid and/or Sodium Citrate. | |||
* Sufficient Indigo Carmine is contained in each 5 mL ampule to permit accurate withdrawal and administration of the full dose. It gives a deep blue solution when dissolved in water. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 235: | Line 107: | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK=* Indigo Carmine is excreted largely by the [[kidneys]], retaining its blue color during passage through the body. | ||
* Elimination of the dye begins soon after injection, appearing in the urine within 10 minutes in average cases. The biological half-life is 4 to 5 minutes following [[intravenous]] injection. Larger quantities are necessary when intramuscular injection is employed. Appearance time and elimination are delayed following [[intramuscular]] injection. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
| Line 244: | Line 118: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* NDC 17478-508-01 | ||
|storage=Store at 20° to 25°C (68° to 77°F) | |||
|packLabel=<!--Patient Counseling Information--> | : 5 mL ampules packaged in boxes of 10. | ||
|storage=* Store at 20° to 25°C (68° to 77°F) | |||
|packLabel=====PRINCIPAL DISPLAY PANEL==== | |||
: [[File:Indigo PDP 1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Indigo PDP 2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File:Indigo I n A.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
| Line 253: | Line 139: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* INDIGO CARMINE®<ref>{{Cite web | title = Indigotindisulfonate sodium | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=482e3688-b942-4661-8ab5-3a0db625a405}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=* | |lookAlike=* There is limited information regarding the look alike drug names. | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
Latest revision as of 16:30, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Indigotindisulfonate is a Diagnostic Agent that is FDA approved for the diagnosis of ureteral orifices during cystoscopy and ureteral catheterization. Common adverse reactions include allergic reaction.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Originally employed as a kidney function test, the chief application of Indigo Carmine at present is localizing ureteral orifices during cystoscopy and ureteral catheterization.
Dosage
- Indigo Carmine solution is injected either by the intravenous or intramuscular route, and its appearance at the ureteral orifices is watched with the cystoscope in place. The intravenous method is preferred because a 5 mL injection is sufficient. A lesser dosage in infants, children and underweight patients will prevent skin coloration.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Indigotindisulfonate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Indigotindisulfonate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy in pediatrics patients has not been established
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Indigotindisulfonate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Indigotindisulfonate in pediatric patients.
Contraindications
- Indigo Carmine is contraindicated in patients who have previously experienced an adverse reaction following its use.
Warnings
- An occasional idiosyncratic drug reaction may occur. A mild pressor effect may be encountered in some patients.
- Since precipitation of indigotindisulfonate sodium may occur, Indigo Carmine Solution must not be diluted prior to injection or injected with infusion assemblies which were used with other solutions.
Precautions
- Indigo Carmine should be stored in the dark, away from direct light, preferably in the original package.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Indigotindisulfonate in the drug label.
Postmarketing Experience
Drug Interactions
There is limited information regarding Indigotindisulfonate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal Reproduction studies have not been conducted with indigotindisulfonate sodium injection. It is also not known whether indigotindisulfonate sodium injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indigotindisulfonate sodium injection should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Indigotindisulfonate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Indigotindisulfonate during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Indigo Carmine is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Indigotindisulfonate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Indigotindisulfonate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Indigotindisulfonate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Indigotindisulfonate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Indigotindisulfonate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Indigotindisulfonate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Indigotindisulfonate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Indigotindisulfonate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Indigotindisulfonate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Indigotindisulfonate in the drug label.
Overdosage
- There are no data available describing the signs, symptoms or laboratory findings accompanying overdosage.
- No discernible symptoms of toxicity have been observed in mice with an intravenous dose of 200 mg/kg. After intravenous administration the LD80 was established at 300 mg/kg in mice.
DRUG ABUSE AND DEPENDENCE
- Indigo Carmine is not a controlled substance listed in any of the Drug Enforcement Administration Schedules. Its use is not known to lead to dependence or abuse.
Pharmacology
There is limited information regarding Indigotindisulfonate Pharmacology in the drug label.
Mechanism of Action
Structure
- Each mL contains:
- Active: Indigotindisulfonate Sodium 8 mg Inactives: Water for injection, q.s. pH adjusted when necessary with Citric Acid and/or Sodium Citrate.
- Sufficient Indigo Carmine is contained in each 5 mL ampule to permit accurate withdrawal and administration of the full dose. It gives a deep blue solution when dissolved in water.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Indigotindisulfonate in the drug label.
Pharmacokinetics
- Indigo Carmine is excreted largely by the kidneys, retaining its blue color during passage through the body.
- Elimination of the dye begins soon after injection, appearing in the urine within 10 minutes in average cases. The biological half-life is 4 to 5 minutes following intravenous injection. Larger quantities are necessary when intramuscular injection is employed. Appearance time and elimination are delayed following intramuscular injection.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Indigotindisulfonate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Indigotindisulfonate in the drug label.
How Supplied
- NDC 17478-508-01
- 5 mL ampules packaged in boxes of 10.
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Indigotindisulfonate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Indigotindisulfonate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Indigotindisulfonate in the drug label.
Precautions with Alcohol
- Alcohol-Indigotindisulfonate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- INDIGO CARMINE®[1]
Look-Alike Drug Names
- There is limited information regarding the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.