Imipenem cilastatin microbiology: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
|||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{imipenem cilastatin}} | ||
{{CMG}} | {{CMG}}; {{AE}} {{SS}} | ||
==Microbiology== | ==Microbiology== | ||
The bactericidal activity of [[Imipenem]] results from the inhibition of cell wall synthesis. Its greatest affinity is for penicillin binding proteins (PBPs) 1A, 1B, 2, 4, 5 and 6 ofEscherichia coli, and 1A, 1B, 2, 4 and 5 of [[Pseudomonas aeruginosa]]. The lethal effect is related to binding to PBP 2 and PBP 1B. | The bactericidal activity of [[Imipenem]] results from the inhibition of cell wall synthesis. Its greatest affinity is for penicillin binding proteins (PBPs) 1A, 1B, 2, 4, 5 and 6 ofEscherichia coli, and 1A, 1B, 2, 4 and 5 of [[Pseudomonas aeruginosa]]. The lethal effect is related to binding to PBP 2 and PBP 1B. | ||
| Line 10: | Line 11: | ||
[[Imipenem]] has in vitro activity against a wide range of gram-positive and gram-negative organisms. [[Imipenem]] has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections treated with the intravenous formulation of [[Imipenem]]-[[cilastatin]] sodium as described in the INDICATIONS AND USAGE section. | [[Imipenem]] has in vitro activity against a wide range of gram-positive and gram-negative organisms. [[Imipenem]] has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections treated with the intravenous formulation of [[Imipenem]]-[[cilastatin]] sodium as described in the INDICATIONS AND USAGE section. | ||
===Gram-Positive Aerobes=== | =====Gram-Positive Aerobes===== | ||
*Enterococcus faecalis (formerlyS. faecalis) | * [[Enterococcus faecalis (formerlyS. faecalis) | ||
(NOTE: [[Imipenem]] is inactive in vitro against [[Enterococcus faecium]] [formerly | (NOTE: [[Imipenem]] is inactive in vitro against [[Enterococcus faecium]] [formerly | ||
S. faecium ].) | S. faecium ].) | ||
*[[Staphylococcus aureus]] including penicillinase-producing strains | * [[Staphylococcus aureus]] including penicillinase-producing strains | ||
*[[Staphylococcus epidermidis]] including penicillinase-producing strains | * [[Staphylococcus epidermidis]] including penicillinase-producing strains | ||
(NOTE: [[Methicillin]]-resistant [[staphylococci]] should be reported as resistant to | (NOTE: [[Methicillin]]-resistant [[staphylococci]] should be reported as resistant to | ||
[[Imipenem]].) | [[Imipenem]].) | ||
*[[Streptococcus agalactiae]] (Group B streptococci) | * [[Streptococcus agalactiae]] (Group B streptococci) | ||
*[[Streptococcus pneumoniae]] | * [[Streptococcus pneumoniae]] | ||
*[[Streptococcus pyogenes]] | * [[Streptococcus pyogenes]] | ||
===Gram-negative aerobes=== | === Gram-negative aerobes === | ||
*[[Acinetobacter]] spp. | * [[Acinetobacter]] spp. | ||
*[[Citrobacter]] spp. | * [[Citrobacter]] spp. | ||
*[[Enterobacter]] spp. | * [[Enterobacter]] spp. | ||
*[[Escherichia coli]] | * [[Escherichia coli]] | ||
*[[Gardnerella vaginalis]] | * [[Gardnerella vaginalis]] | ||
*[[Haemophilus influenzae]] | * [[Haemophilus influenzae]] | ||
*[[Haemophilus parainfluenzae]] | * [[Haemophilus parainfluenzae]] | ||
*[[Klebsiella]] spp. | * [[Klebsiella]] spp. | ||
*[[Morganella morganii]] | * [[Morganella morganii]] | ||
*[[Proteus vulgaris]] | * [[Proteus vulgaris]] | ||
*[[Providencia rettgeri]] | * [[Providencia rettgeri]] | ||
*[[Pseudomonas aeruginosa]] | * [[Pseudomonas aeruginosa]] | ||
(NOTE: [[Imipenem]] is inactive in vitro against [[Stenotrophomonas]] [formerly Xanthomonas, formerly Pseudomonas] [[maltophilia]] and some strains of Burkholderia cepacia.) | (NOTE: [[Imipenem]] is inactive in vitro against [[Stenotrophomonas]] [formerly Xanthomonas, formerly Pseudomonas] [[maltophilia]] and some strains of Burkholderia cepacia.) | ||
*[[Serratia]] spp., including S. [[marcescens]] | * [[Serratia]] spp., including S. [[marcescens]] | ||
===Gram-positive anaerobes=== | ===Gram-positive anaerobes=== | ||
*[[Bifidobacterium]] spp. | * [[Bifidobacterium]] spp. | ||
*[[Clostridium]] spp. | * [[Clostridium]] spp. | ||
*[[Eubacterium]] spp. | * [[Eubacterium]] spp. | ||
*[[Peptococcus]] spp. | * [[Peptococcus]] spp. | ||
*[[Peptostreptococcus]] spp. | * [[Peptostreptococcus]] spp. | ||
*[[Propionibacterium]] spp. | * [[Propionibacterium]] spp. | ||
===Gram-negative anaerobes=== | ===Gram-negative anaerobes=== | ||
*[[Bacteroides]] spp., including B. | * [[Bacteroides]] spp., including [[B. fragilis]] | ||
*[[Fusobacterium]] spp. | * [[Fusobacterium]] spp. | ||
The following in vitro data are available, but their clinical significance is unknown. | The following in vitro data are available, but their clinical significance is unknown. | ||
| Line 58: | Line 59: | ||
[[Imipenem]] exhibits in vitro minimum inhibitory concentrations (MICs) of 4 µg/mL or less against most (≥90%) strains of the following microorganisms; however, the safety and effectiveness of [[Imipenem]] in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials. | [[Imipenem]] exhibits in vitro minimum inhibitory concentrations (MICs) of 4 µg/mL or less against most (≥90%) strains of the following microorganisms; however, the safety and effectiveness of [[Imipenem]] in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials. | ||
===Gram-positive aerobes=== | ===Gram-positive aerobes=== | ||
*[[Bacillus]] spp. | * [[Bacillus]] spp. | ||
*[[Listeria monocytogenes]] | * [[Listeria monocytogenes]] | ||
*[[Nocardia]] spp. | * [[Nocardia]] spp. | ||
*[[Staphylococcus saprophyticus]] | * [[Staphylococcus saprophyticus]] | ||
*Group C [[streptococci]] | * Group C [[streptococci]] | ||
*Group G [[streptococci]] | * Group G [[streptococci]] | ||
*Viridans group [[streptococci]] | * Viridans group [[streptococci]] | ||
===Gram-negative aerobes=== | ===Gram-negative aerobes=== | ||
*[[Aeromonas hydrophila]] | * [[Aeromonas hydrophila]] | ||
*[[Alcaligenes]]spp. | * [[Alcaligenes]]spp. | ||
*[[Capnocytophaga | * [[Capnocytophaga]] spp. | ||
*[[Haemophilus ducreyi]] | * [[Haemophilus ducreyi]] | ||
*[[Neisseria gonorrhoeae]] including penicillinase-producing strains | * [[Neisseria gonorrhoeae]] including penicillinase-producing strains | ||
*[[Pasteurella]] spp. | * [[Pasteurella]] spp. | ||
*[[Providencia stuartii]] | * [[Providencia stuartii]] | ||
===Gram-negative anaerobes=== | ===Gram-negative anaerobes=== | ||
*[[Prevotella bivia]] | * [[Prevotella bivia]] | ||
*[[Prevotella disiens]] | * [[Prevotella disiens]] | ||
*[[Prevotella melaninogenica]] | * [[Prevotella melaninogenica]] | ||
*[[Veillonella]] spp. | * [[Veillonella]] spp. | ||
In vitro tests show [[Imipenem]] to act synergistically with [[aminoglycoside]] antibiotics against some isolates of [[Pseudomonas aeruginosa]]. | In vitro tests show [[Imipenem]] to act synergistically with [[aminoglycoside]] antibiotics against some isolates of [[Pseudomonas aeruginosa]]. | ||
| Line 105: | Line 106: | ||
{| | {| | ||
|- | |- | ||
|[[File:Imipenem Cilastatin 3.jpg|thumb| | |[[File:Imipenem Cilastatin 3.jpg|thumb|800px|left]] | ||
|- | |- | ||
|} | |} | ||
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound at the infection site reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound at the infection site reaches the concentrations usually achievable, and that other therapy should be selected. | A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound at the infection site reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound at the infection site reaches the concentrations usually achievable, and that other therapy should be selected. | ||
| Line 118: | Line 118: | ||
{| | {| | ||
|- | |- | ||
|[[File:Imipenem Cilastatin 4.jpg|thumb| | |[[File:Imipenem Cilastatin 4.jpg|thumb|800px|left]] | ||
|- | |- | ||
|} | |} | ||
Latest revision as of 22:32, 5 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Microbiology
The bactericidal activity of Imipenem results from the inhibition of cell wall synthesis. Its greatest affinity is for penicillin binding proteins (PBPs) 1A, 1B, 2, 4, 5 and 6 ofEscherichia coli, and 1A, 1B, 2, 4 and 5 of Pseudomonas aeruginosa. The lethal effect is related to binding to PBP 2 and PBP 1B.
Imipenem has a high degree of stability in the presence of beta-lactamases, both penicillinases and cephalosporinases produced by gram-negative and gram-positive bacteria. It is a potent inhibitor of beta-lactamases from certain gram-negative bacteria which are inherently resistant to most beta-lactam antibiotics, e.g.,Pseudomonas aeruginosa, Serratia spp., and Enterobacter spp.
Imipenem has in vitro activity against a wide range of gram-positive and gram-negative organisms. Imipenem has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections treated with the intravenous formulation of Imipenem-cilastatin sodium as described in the INDICATIONS AND USAGE section.
Gram-Positive Aerobes
- [[Enterococcus faecalis (formerlyS. faecalis)
(NOTE: Imipenem is inactive in vitro against Enterococcus faecium [formerly S. faecium ].)
- Staphylococcus aureus including penicillinase-producing strains
- Staphylococcus epidermidis including penicillinase-producing strains
(NOTE: Methicillin-resistant staphylococci should be reported as resistant to Imipenem.)
- Streptococcus agalactiae (Group B streptococci)
- Streptococcus pneumoniae
- Streptococcus pyogenes
Gram-negative aerobes
- Acinetobacter spp.
- Citrobacter spp.
- Enterobacter spp.
- Escherichia coli
- Gardnerella vaginalis
- Haemophilus influenzae
- Haemophilus parainfluenzae
- Klebsiella spp.
- Morganella morganii
- Proteus vulgaris
- Providencia rettgeri
- Pseudomonas aeruginosa
(NOTE: Imipenem is inactive in vitro against Stenotrophomonas [formerly Xanthomonas, formerly Pseudomonas] maltophilia and some strains of Burkholderia cepacia.)
- Serratia spp., including S. marcescens
Gram-positive anaerobes
- Bifidobacterium spp.
- Clostridium spp.
- Eubacterium spp.
- Peptococcus spp.
- Peptostreptococcus spp.
- Propionibacterium spp.
Gram-negative anaerobes
- Bacteroides spp., including B. fragilis
- Fusobacterium spp.
The following in vitro data are available, but their clinical significance is unknown.
Imipenem exhibits in vitro minimum inhibitory concentrations (MICs) of 4 µg/mL or less against most (≥90%) strains of the following microorganisms; however, the safety and effectiveness of Imipenem in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Gram-positive aerobes
- Bacillus spp.
- Listeria monocytogenes
- Nocardia spp.
- Staphylococcus saprophyticus
- Group C streptococci
- Group G streptococci
- Viridans group streptococci
Gram-negative aerobes
- Aeromonas hydrophila
- Alcaligenesspp.
- Capnocytophaga spp.
- Haemophilus ducreyi
- Neisseria gonorrhoeae including penicillinase-producing strains
- Pasteurella spp.
- Providencia stuartii
Gram-negative anaerobes
In vitro tests show Imipenem to act synergistically with aminoglycoside antibiotics against some isolates of Pseudomonas aeruginosa.
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide to the physician the results of in vitro susceptibility tests for antimicrobial drug products used in resident hospitals as periodic reports which describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a broth dilution method{1,2} or equivalent with standardized inoculum concentrations and standardized concentrations of Imipenem powder. The MIC values should be interpreted according to criteria provided in Table below.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure requires the use of standardized inoculum concentrations {2,3}. This procedure uses paper disks impregnated with 10-µg Imipenem to test the susceptibility of microorganisms to Imipenem. The disk diffusion interpretive criteria should be interpreted according to criteria provided in Table below.
Anaerobic Techniques
For anaerobic bacteria, the susceptibility to Imipenem as MICs can be determined by standardized test methods.{2,4} The MIC values obtained should be interpreted according to criteria provided in Table below.
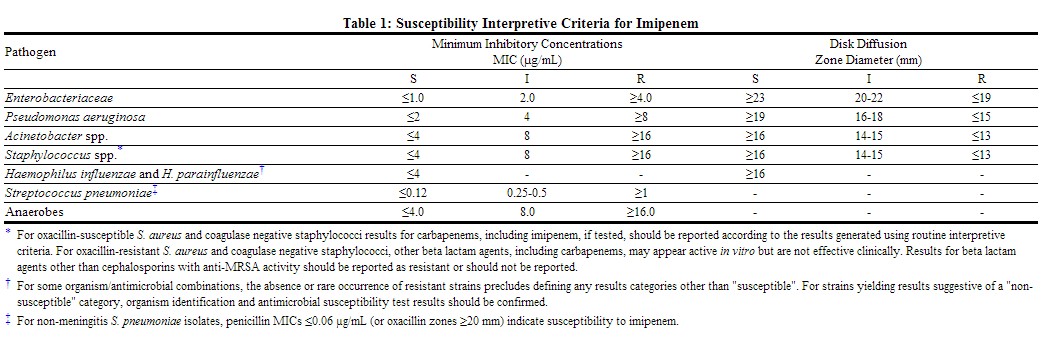 |
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound at the infection site reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound at the infection site reaches the concentrations usually achievable, and that other therapy should be selected.
Quality Control
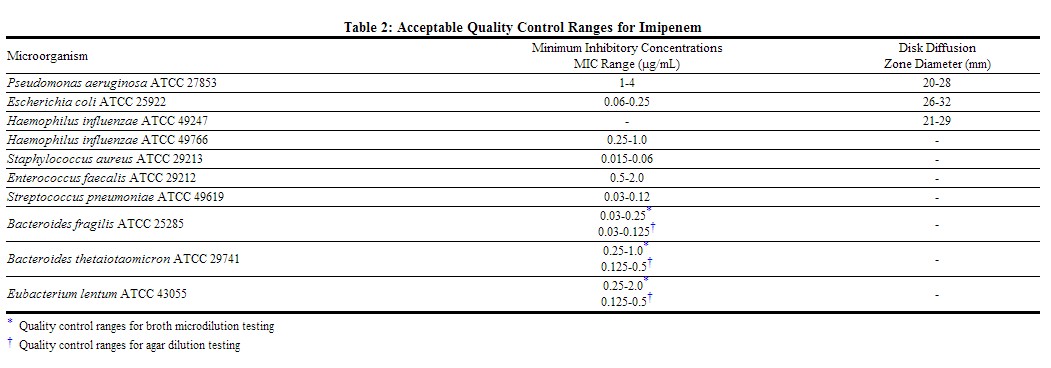
Standardized susceptibility test procedures require the use of laboratory control microorganisms to ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test. Quality control microorganisms are specific strains of organisms with intrinsic biological properties. QC strains are very stable strains which will give a standard and repeatable susceptibility pattern. The specific strains used for microbiological quality control are not clinically significant. Standard imipenem powder should provide the following range of values noted in Table below.[1]
 |
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050587s065,050630s028lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.