Imatinib: Difference between revisions
No edit summary |
No edit summary |
||
| Line 579: | Line 579: | ||
|structure= | |structure= | ||
* Imatinib is a small molecule kinase inhibitor. Gleevec film-coated tablets contain imatinib mesylate equivalent to 100 mg or 400 mg of imatinib free base. Imatinib mesylate is designated chemically as 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3- | * Imatinib is a small molecule kinase inhibitor. Gleevec film-coated tablets contain imatinib mesylate equivalent to 100 mg or 400 mg of imatinib free base. Imatinib mesylate is designated chemically as 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate and its structural formula is | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
Revision as of 00:54, 4 August 2014
{{DrugProjectFormSinglePage |authorTag=
Vignesh Ponnusamy, M.B.B.S. [1]
|genericName=
Imatinib
|aOrAn=
a
|drugClass=
protein-tyrosine kinase inhibitor
|indication=
newly diagnosed philadelphia positive chronic myeloid leukemia (ph+ CML), ph+ CML in blast crisis (bc), accelerated phase (ap) or chronic phase (cp) after interferon-alpha (ifn) therapy, adult patients with ph+ acute lymphoblastic leukemia (ALL), pediatric patients with ph+ acute lymphoblastic leukemia (ALL), myelodysplastic/myeloproliferative diseases (MDS/MPD), aggressive systemic mastocytosis (ASM), hypereosinophilic syndrome (HES) and/or chronic eosinophilic leukemia (CEL), dermatofibrosarcoma protuberans (DFSP), kit+ gastrointestinal stromal tumors (GIST), adjuvant treatment of GIST
|hasBlackBoxWarning=
|adverseReactions=
edema, nausea, vomiting, muscle cramps, musculoskeletal pain, diarrhea, rash, fatigue and abdominal pain
|blackBoxWarningTitle=
Title
|blackBoxWarningBody= ConditionName:
- Content
|fdaLIADAdult=
Newly Diagnosed Philadelphia Positive Chronic Myeloid Leukemia (Ph+ CML)
- Newly diagnosed adult patients with Philadelphia chromosome positive chronic myeloid leukemia in chronic phase.
Ph+ CML in Blast Crisis (BC), Accelerated Phase (AP) or Chronic Phase (CP) After Interferon-alpha (IFN) Therapy
- Patients with Philadelphia chromosome positive chronic myeloid leukemia in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy.
Adult patients with Ph+ Acute Lymphoblastic Leukemia (ALL)
- Adult patients with relapsed or refractory Philadelphia chromosome positive acute lymphoblastic leukemia.
Myelodysplastic/Myeloproliferative Diseases (MDS/MPD)
- Adult patients with myelodysplastic/ myeloproliferative diseases associated with PDGFR (platelet-derived growth factor receptor) gene re-arrangements.
Aggressive Systemic Mastocytosis (ASM)
- Adult patients with aggressive systemic mastocytosis without the D816V c-Kit mutation or with c-Kit mutational status unknown.
Hypereosinophilic Syndrome (HES) and/or Chronic Eosinophilic Leukemia (CEL)
- Adult patients with hypereosinophilic syndrome and/or chronic eosinophilic leukemia who have the FIP1L1-PDGFRα fusion kinase (mutational analysis or FISH demonstration of CHIC2 allele deletion) and for patients with HES and/or CEL who are FIP1L1-PDGFRα fusion kinase negative or unknown.
Dermatofibrosarcoma Protuberans (DFSP)
- Adult patients with unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans.
Kit+ Gastrointestinal Stromal Tumors (GIST)
- Patients with Kit (CD117) positive unresectable and/or metastatic malignant gastrointestinal stromal tumors.
Adjuvant Treatment of GIST
- Adjuvant treatment of adult patients following complete gross resection of Kit (CD117) positive GIST.
Dosage And Administration
- Therapy should be initiated by a physician experienced in the treatment of patients with hematological malignancies or malignant sarcomas, as appropriate. The prescribed dose should be administered orally, with a meal and a large glass of water. Doses of 400 mg or 600 mg should be administered once daily, whereas a dose of 800 mg should be administered as 400 mg twice a day.
- In children, Gleevec treatment can be given as a once-daily dose in CML and Ph+ ALL. Alternatively, in children with CML the daily dose may be split into two - one portion dosed in the morning and one portion in the evening. There is no experience with Gleevec treatment in children under 1 year of age.
- For patients unable to swallow the film-coated tablets, the tablets may be dispersed in a glass of water or apple juice. The required number of tablets should be placed in the appropriate volume of beverage (approximately 50 mL for a 100 mg tablet, and 200 mL for a 400 mg tablet) and stirred with a spoon. The suspension should be administered immediately after complete disintegration of the tablet(s).
- For daily dosing of 800 mg and above, dosing should be accomplished using the 400 mg tablet to reduce exposure to iron.
- Treatment may be continued as long as there is no evidence of progressive disease or unacceptable toxicity.
Adult Patients with Ph+ CML CP, AP, and BC
- The recommended dose of Gleevec is 400 mg/day for adult patients in chronic phase CML and 600 mg/day for adult patients in accelerated phase or blast crisis.
- In CML, a dose increase from 400 mg to 600 mg in adult patients with chronic phase disease, or from 600 mg to 800 mg (given as 400 mg twice daily) in adult patients in accelerated phase or blast crisis may be considered in the absence of severe adverse drug reaction and severe non-leukemia related neutropenia or thrombocytopenia in the following circumstances: disease progression (at any time), failure to achieve a satisfactory hematologic response after at least 3 months of treatment, failure to achieve a cytogenetic response after 6-12 months of treatment, or loss of a previously achieved hematologic or cytogenetic response.
2.2 Pediatric Patients with Ph+ CML CP
The recommended dose of Gleevec for children with newly diagnosed Ph+ CML is 340 mg/m2/day (not to exceed 600 mg).
Adults Patients with Ph+ ALL
- The recommended dose of Gleevec is 600 mg/day for adult patients with relapsed/refractory Ph+ ALL.
2.4 Pediatric Patients with Ph+ ALL
The recommended dose of Gleevec to be given in combination with chemotherapy to children with newly diagnosed Ph+ ALL is 340mg/m2/day (not to exceed 600mg).
MDS/MPD
- The recommended dose of Gleevec is 400 mg/day for adult patients with MDS/MPD.
ASM
- The recommended dose of Gleevec is 400 mg/day for adult patients with ASM without the D816V c-Kit mutation. If c-Kit mutational status is not known or unavailable, treatment with Gleevec 400 mg/day may be considered for patients with ASM not responding satisfactorily to other therapies. For patients with ASM associated with eosinophilia, a clonal hematological disease related to the fusion kinase FIP1L1-PDGFRα, a starting dose of 100 mg/day is recommended. Dose increase from 100 mg to 400 mg for these patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy.
HES/CEL
- The recommended dose of Gleevec is 400 mg/day for adult patients with HES/CEL. For HES/CEL patients with demonstrated FIP1L1-PDGFRα fusion kinase, a starting dose of 100 mg/day is recommended. Dose increase from 100 mg to 400 mg for these patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy.
DFSP
- The recommended dose of Gleevec is 800 mg/day for adult patients with DFSP.
Metastatic or Unresectable GIST
- The recommended dose of Gleevec is 400 mg/day for adult patients with unresectable and/or metastatic, malignant GIST. A dose increase up to 800 mg daily (given as 400 mg twice daily) may be considered, as clinically indicated, in patients showing clear signs or symptoms of disease progression at a lower dose and in the absence of severe adverse drug reactions.
Adjuvant GIST
- The recommended dose of Gleevec is 400 mg/day for the adjuvant treatment of adult patients following complete gross resection of GIST. In clinical trials one year of Gleevec and three years of Gleevec were studied. In the patient population defined in Study 2, three years of Gleevec is recommended [see Clinical Studies (14.8)]. The optimal treatment duration with Gleevec is not known.
Dose Modification Guidelines
- Concomitant Strong CYP3A4 inducers: The use of concomitant strong CYP3A4 inducers should be avoided (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifampacin, phenobarbital). If patients must be co-administered a strong CYP3A4 inducer, based on pharmacokinetic studies, the dosage of Gleevec should be increased by at least 50%, and clinical response should be carefully monitored [see Drug Interactions (7.1)].
- Hepatic Impairment: Patients with mild and moderate hepatic impairment do not require a dose adjustment and should be treated per the recommended dose. A 25% decrease in the recommended dose should be used for patients with severe hepatic impairment [see Use in Specific Populations (8.6)].
- Renal Impairment: Patients with moderate renal impairment (CrCL=20-39 mL/min) should receive a 50% decrease in the recommended starting dose and future doses can be increased as tolerated. Doses greater than 600 mg are not recommended in patients with mild renal impairment (CrCL=40-59 mL/min). For patients with moderate renal impairment doses greater than 400 mg are not recommended.
- Imatinib should be used with caution in patients with severe renal impairment. A dose of 100 mg/day was tolerated in two patients with severe renal impairment [See Warnings and Precautions (5.3), Use in Specific Populations (8.7)].
Dose Adjustment for Hepatotoxicity and Non-Hematologic Adverse Reactions
- If elevations in bilirubin >3 x institutional upper limit of normal (IULN) or in liver transaminases >5 x IULN occur, Gleevec should be withheld until bilirubin levels have returned to a <1.5 x IULN and transaminase levels to <2.5 x IULN. In adults, treatment with Gleevec may then be continued at a reduced daily dose (i.e., 400 mg to 300 mg, 600 mg to 400 mg or 800 mg to 600 mg). In children, daily doses can be reduced under the same circumstances from 340 mg/m2/day to 260 mg/m2/day.
- If a severe non-hematologic adverse reaction develops (such as severe hepatotoxicity or severe fluid retention), Gleevec should be withheld until the event has resolved. Thereafter, treatment can be resumed as appropriate depending on the initial severity of the event.
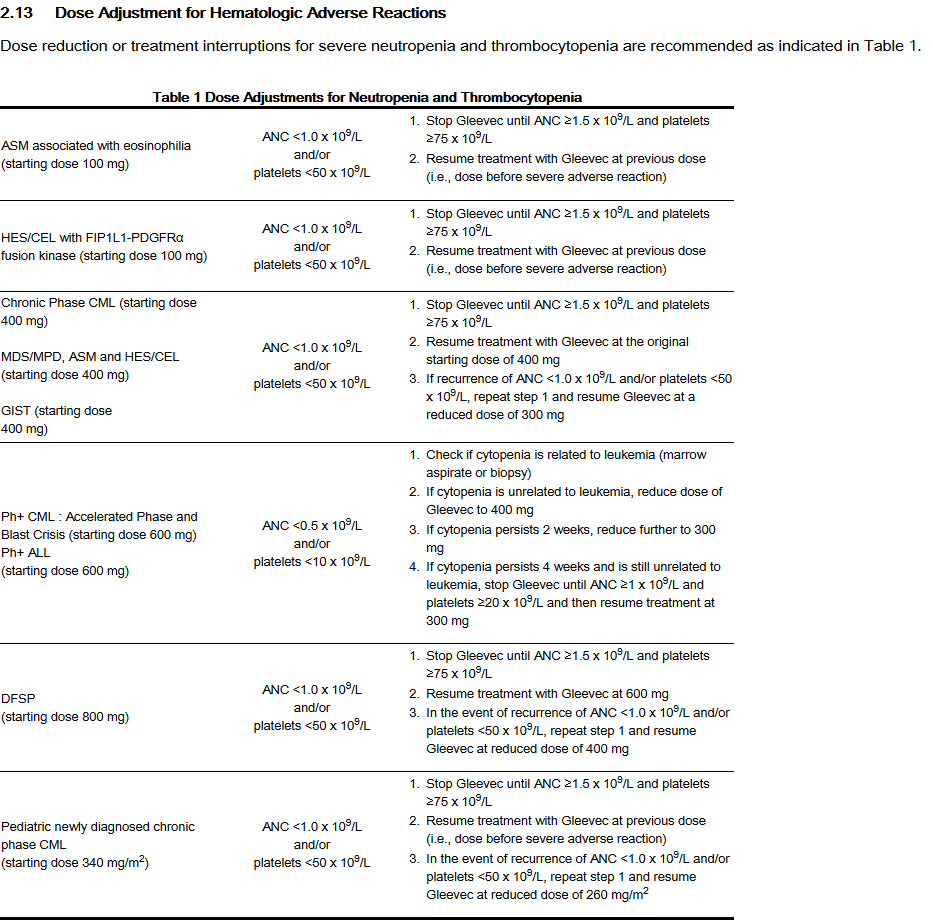
Dose Adjustment for Hematologic Adverse Reactions
- Dose reduction or treatment interruptions for severe neutropenia and thrombocytopenia are recommended as indicated in Table 1.
|offLabelAdultGuideSupport=
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Imatinib in adult patients.
|offLabelAdultNoGuideSupport=
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Imatinib in adult patients.
|fdaLIADPed=
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Imatinib in pediatric patients.
|offLabelPedGuideSupport=
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Imatinib in pediatric patients.
|offLabelPedNoGuideSupport=
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Imatinib in pediatric patients.
|contraindications=
- Condition1
|warnings=
- Description
Precautions
- Description
|clinicalTrials=
There is limited information regarding Clinical Trial Experience of Imatinib in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
|postmarketing=
There is limited information regarding Postmarketing Experience of Imatinib in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
|drugInteractions=
- Drug
- Description
|useInPregnancyFDA=
- Pregnancy Category
|useInPregnancyAUS=
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Imatinib in women who are pregnant.
|useInLaborDelivery= There is no FDA guidance on use of Imatinib during labor and delivery.
|useInNursing= There is no FDA guidance on the use of Imatinib with respect to nursing mothers.
|useInPed= There is no FDA guidance on the use of Imatinib with respect to pediatric patients.
|useInGeri= There is no FDA guidance on the use of Imatinib with respect to geriatric patients.
|useInGender= There is no FDA guidance on the use of Imatinib with respect to specific gender populations.
|useInRace= There is no FDA guidance on the use of Imatinib with respect to specific racial populations.
|useInRenalImpair= There is no FDA guidance on the use of Imatinib in patients with renal impairment.
|useInHepaticImpair= There is no FDA guidance on the use of Imatinib in patients with hepatic impairment.
|useInReproPotential= There is no FDA guidance on the use of Imatinib in women of reproductive potentials and males.
|useInImmunocomp= There is no FDA guidance one the use of Imatinib in patients who are immunocompromised.
|administration=
- Oral
- Intravenous
|monitoring=
There is limited information regarding Monitoring of Imatinib in the drug label.
- Description
|IVCompat=
There is limited information regarding IV Compatibility of Imatinib in the drug label.
|overdose=
Acute Overdose
Signs and Symptoms
- Adult Overdose
- 1,200 to 1,600 mg (duration varying between 1 to 10 days): Nausea, vomiting, diarrhea, rash erythema, edema, swelling, fatigue, muscle spasms, thrombocytopenia, pancytopenia, abdominal pain, headache, decreased appetite.
- 1,800 to 3,200 mg (as high as 3,200 mg daily for 6 days): Weakness, myalgia, increased CPK, increased bilirubin, gastrointestinal pain.
- 6,400 mg (single dose): One case in the literature reported one patient who experienced nausea, vomiting, abdominal pain, pyrexia, facial swelling, neutrophil count decreased, increase transaminases.
- 8 to 10 g (single dose): Vomiting and gastrointestinal pain have been reported.
- A patient with myeloid blast crisis experienced Grade 1 elevations of serum creatinine, Grade 2 ascites and elevated liver transaminase levels, and Grade 3 elevations of bilirubin after inadvertently taking 1,200 mg of Gleevec daily for 6 days. Therapy was temporarily interrupted and complete reversal of all abnormalities occurred within 1 week. Treatment was resumed at a dose of 400 mg daily without recurrence of adverse reactions. Another patient developed severe muscle cramps after taking 1,600 mg of Gleevec daily for 6 days. Complete resolution of muscle cramps occurred following interruption of therapy and treatment was subsequently resumed. Another patient that was prescribed 400 mg daily, took 800 mg of Gleevec on Day 1 and 1,200 mg on Day 2. Therapy was interrupted, no adverse reactions occurred and the patient resumed therapy.
- Pediatric Overdose
- One 3 year-old male exposed to a single dose of 400 mg experienced vomiting, diarrhea and anorexia and another 3 year-old male exposed to a single dose of 980 mg experienced decreased white blood cell count and diarrhea.
Management
- In the event of overdosage, the patient should be observed and appropriate supportive treatment given.
Chronic Overdose
There is limited information regarding Chronic Overdose of Imatinib in the drug label.
|drugBox=
{{drugbox2 | verifiedrevid = 459451238 | IUPAC_name = 4-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide | image = Imatinib.png | width = 300 | image2 = Imatinib1.gif | width2 = 250 | tradename = Gleevec, Glivec | Drugs.com = Monograph | MedlinePlus = a606018 | licence_EU = Glivec | licence_US = IMATINIB | pregnancy_AU = D | pregnancy_US = D | legal_AU = S4 | legal_CA = Rx-only | legal_UK = POM | legal_US = Rx-only | routes_of_administration = Oral
| bioavailability = 98%
| protein_bound = 95%
| metabolism = Hepatic (mainly CYP3A4-mediated)
| elimination_half-life = 18 hours (imatinib)
40 hours (active metabolite)
| excretion = Faecal (68%) and renal (13%)
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 152459-95-5
| CAS_supplemental =
220127-57-1 (mesilate)
| ATC_prefix = L01
| ATC_suffix = XE01
| PubChem = 5291
| DrugBank_Ref =
| DrugBank = DB00619
| ChemSpiderID_Ref =
| ChemSpiderID = 5101
| UNII_Ref =
| UNII = BKJ8M8G5HI
| KEGG_Ref =
| KEGG = D08066
| ChEBI_Ref =
| ChEBI = 45783
| ChEMBL_Ref =
| ChEMBL = 941
| C=29 | H=31 | N=7 | O=1
| molecular_weight = 493.603 g/mol
589.7 g/mol (mesilate)
| smiles = Cc1ccc(cc1Nc2nccc(n2)c3cccnc3)NC(=O)c4ccc(cc4)CN5CCN(CC5)C
| InChI = 1/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34)
| InChIKey = KTUFNOKKBVMGRW-UHFFFAOYAJ
| StdInChI_Ref =
| StdInChI = 1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34)
| StdInChIKey_Ref =
| StdInChIKey = KTUFNOKKBVMGRW-UHFFFAOYSA-N
}}
|mechAction=
- Imatinib mesylate is a protein-tyrosine kinase inhibitor that inhibits the bcr-abl tyrosine kinase, the constitutive abnormal tyrosine kinase created by the Philadelphia chromosome abnormality in CML. Imatinib inhibits proliferation and induces apoptosis in bcr-abl positive cell lines as well as fresh leukemic cells from Philadelphia chromosome positive chronic myeloid leukemia. Imatinib inhibits colony formation in assays using ex vivo peripheral blood and bone marrow samples from CML patients.
- In vivo, imatinib inhibits tumor growth of bcr-abl transfected murine myeloid cells as well as bcr-abl positive leukemia lines derived from CML patients in blast crisis.
- Imatinib is also an inhibitor of the receptor tyrosine kinases for platelet-derived growth factor (PDGF) and stem cell factor (SCF), c-kit, and inhibits PDGF- and SCF-mediated cellular events. In vitro, imatinib inhibits proliferation and induces apoptosis in GIST cells, which express an activating c-kit mutation.
|structure=
- Imatinib is a small molecule kinase inhibitor. Gleevec film-coated tablets contain imatinib mesylate equivalent to 100 mg or 400 mg of imatinib free base. Imatinib mesylate is designated chemically as 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate and its structural formula is
- Imatinib mesylate is a white to off-white to brownish or yellowish tinged crystalline powder. Its molecular formula is C29H31N7O • CH4SO3 and its molecular weight is 589.7. Imatinib mesylate is soluble in aqueous buffers ≤pH 5.5 but is very slightly soluble to insoluble in neutral/alkaline aqueous buffers. In non-aqueous solvents, the drug substance is freely soluble to very slightly soluble in dimethyl sulfoxide, methanol, and ethanol, but is insoluble in n-octanol, acetone, and acetonitrile.
- Inactive Ingredients: colloidal silicon dioxide (NF); crospovidone (NF); hydroxypropyl methylcellulose (USP); magnesium stearate (NF); and microcrystalline cellulose (NF). Tablet coating: ferric oxide, red (NF); ferric oxide, yellow (NF); hydroxypropyl methylcellulose (USP); polyethylene glycol (NF) and talc (USP).
|PD=
There is limited information regarding Pharmacodynamics of Imatinib in the drug label.
|PK=
- The pharmacokinetics of Gleevec have been evaluated in studies in healthy subjects and in population pharmacokinetic studies in over 900 patients. The pharmacokinetics of Gleevec are similar in CML and GIST patients. Imatinib is well absorbed after oral administration with Cmax achieved within 2-4 hours post-dose. Mean absolute bioavailability is 98%. Following oral administration in healthy volunteers, the elimination half-lives of imatinib and its major active metabolite, the N-demethyl derivative (CGP74588), are approximately 18 and 40 hours, respectively. Mean imatinib AUC increases proportionally with increasing doses ranging from 25 mg-1,000 mg. There is no significant change in the pharmacokinetics of imatinib on repeated dosing, and accumulation is 1.5- to 2.5-fold at steady state when Gleevec is dosed once daily. At clinically relevant concentrations of imatinib, binding to plasma proteins in in vitro experiments is approximately 95%, mostly to albumin and α1-acid glycoprotein.
- CYP3A4 is the major enzyme responsible for metabolism of imatinib. Other cytochrome P450 enzymes, such as CYP1A2, CYP2D6, CYP2C9, and CYP2C19, play a minor role in its metabolism. The main circulating active metabolite in humans is the N-demethylated piperazine derivative, formed predominantly by CYP3A4. It shows in vitro potency similar to the parent imatinib. The plasma AUC for this metabolite is about 15% of the AUC for imatinib. The plasma protein binding of N-demethylated metabolite CGP74588 is similar to that of the parent compound. Human liver microsome studies demonstrated that Gleevec is a potent competitive inhibitor of CYP2C9, CYP2D6, and CYP3A4/5 with Ki values of 27, 7.5, and 8 µM, respectively.
- Imatinib elimination is predominately in the feces, mostly as metabolites. Based on the recovery of compound(s) after an oral 14C-labeled dose of imatinib, approximately 81% of the dose was eliminated within 7 days, in feces (68% of dose) and urine (13% of dose). Unchanged imatinib accounted for 25% of the dose (5% urine, 20% feces), the remainder being metabolites.
- Typically, clearance of imatinib in a 50-year-old patient weighing 50 kg is expected to be 8 L/h, while for a 50-year-old patient weighing 100 kg the clearance will increase to 14 L/h. The inter-patient variability of 40% in clearance does not warrant initial dose adjustment based on body weight and/or age but indicates the need for close monitoring for treatment-related toxicity.
|nonClinToxic=
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In the 2-year rat carcinogenicity study administration of imatinib at 15, 30, and 60 mg/kg/day resulted in a statistically significant reduction in the longevity of males at 60 mg/kg/day and females at ≥30 mg/kg/day. Target organs for neoplastic changes were the kidneys (renal tubule and renal pelvis), urinary bladder, urethra, preputial and clitoral gland, small intestine, parathyroid glands, adrenal glands and non-glandular stomach. Neoplastic lesions were not seen at: 30 mg/kg/day for the kidneys, urinary bladder, urethra, small intestine, parathyroid glands, adrenal glands and non-glandular stomach, and 15 mg/kg/day for the preputial and clitoral gland. The papilloma/carcinoma of the preputial/clitoral gland were noted at 30 and 60 mg/kg/day, representing approximately 0.5 to 4 or 0.3 to 2.4 times the human daily exposure (based on AUC) at 400 mg/day or 800 mg/day, respectively, and 0.4 to 3.0 times the daily exposure in children (based on AUC) at 340 mg/m2. The renal tubule adenoma/carcinoma, renal pelvis transitional cell neoplasms, the urinary bladder and urethra transitional cell papillomas, the small intestine adenocarcinomas, the parathyroid glands adenomas, the benign and malignant medullary tumors of the adrenal glands and the non-glandular stomach papillomas/carcinomas were noted at 60 mg/kg/day. The relevance of these findings in the rat carcinogenicity study for humans is not known.
- Positive genotoxic effects were obtained for imatinib in an in vitro mammalian cell assay (Chinese hamster ovary) for clastogenicity (chromosome aberrations) in the presence of metabolic activation. Two intermediates of the manufacturing process, which are also present in the final product, are positive for mutagenesis in the Ames assay. One of these intermediates was also positive in the mouse lymphoma assay. Imatinib was not genotoxic when tested in an in vitro bacterial cell assay (Ames test), an in vitro mammalian cell assay (mouse lymphoma) and an in vivo rat micronucleus assay.
- In a study of fertility, male rats were dosed for 70 days prior to mating and female rats were dosed 14 days prior to mating and through to gestational Day 6. Testicular and epididymal weights and percent motile sperm were decreased at 60 mg/kg, approximately three-fourths the maximum clinical dose of 800 mg/day based on body surface area. This was not seen at doses ≤20 mg/kg (one-fourth the maximum human dose of 800 mg). The fertility of male and female rats was not affected.
- In a pre- and post-natal development study in female rats dosed with imatinib mesylate at 45 mg/kg (approximately one-half the maximum human dose of 800 mg/day, based on body surface area) from gestational Day 6 until the end of lactation, red vaginal discharge was noted on either gestational Day 14 or 15. In the first generation offspring at this same dose level, mean body weights were reduced from birth until terminal sacrifice. First generation offspring fertility was not affected but reproductive effects were noted at 45 mg/kg/day including an increased number of resorptions and a decreased number of viable fetuses.
- Fertility was not affected in the preclinical fertility and early embryonic development study although lower testes and epididymal weights as well as a reduced number of motile sperm were observed in the high dose males rats. In the preclinical pre- and postnatal study in rats, fertility in the first generation offspring was also not affected by Gleevec.
- Human studies on male patients receiving Gleevec and its affect on male fertility and spermatogenesis have not been performed. Male patients concerned about their fertility on Gleevec treatment should consult with their physician.
|clinicalStudies=
Chronic Myeloid Leukemia
- Chronic Phase, Newly Diagnosed: An open-label, multicenter, international randomized Phase 3 study has been conducted in patients with newly diagnosed Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML) in chronic phase. This study compared treatment with either single-agent Gleevec or a combination of interferon-alpha (IFN) plus cytarabine (Ara-C). Patients were allowed to cross over to the alternative treatment arm if they failed to show a complete hematologic response (CHR) at 6 months, a major cytogenetic response (MCyR) at 12 months, or if they lost a CHR or MCyR. Patients with increasing WBC or severe intolerance to treatment were also allowed to cross over to the alternative treatment arm with the permission of the study monitoring committee (SMC). In the Gleevec arm, patients were treated initially with 400 mg daily. Dose escalations were allowed from 400 mg daily to 600 mg daily, then from 600 mg daily to 800 mg daily. In the IFN arm, patients were treated with a target dose of IFN of 5 MIU/m2/day subcutaneously in combination with subcutaneous Ara-C 20 mg/m2/day for 10 days/month.
- A total of 1,106 patients were randomized from 177 centers in 16 countries, 553 to each arm. Baseline characteristics were well balanced between the two arms. Median age was 51 years (range 18-70 years), with 21.9% of patients ≥60 years of age. There were 59% males and 41% females; 89.9% Caucasian and 4.7% Black patients. At the cut-off for this analysis (7 years after last patient had been recruited), the median duration of first-line treatment was 82 and 8 months in the Gleevec and IFN arm, respectively. The median duration of second-line treatment with Gleevec was 64 months. Sixty percent of patients randomized to Gleevec are still receiving first-line treatment. In these patients, the average dose of Gleevec was 403 mg ± 57 mg. Overall, in patients receiving first line Gleevec, the average daily dose delivered was 406 mg ± 76 mg. Due to discontinuations and cross-overs, only 2% of patients randomized to IFN were still on first-line treatment. In the IFN arm, withdrawal of consent (14%) was the most frequent reason for discontinuation of first-line therapy, and the most frequent reason for cross over to the Gleevec arm was severe intolerance to treatment (26%) and progression (14%).
- The primary efficacy endpoint of the study was progression-free survival (PFS). Progression was defined as any of the following events: progression to accelerated phase or blast crisis (AP/BC), death, loss of CHR or MCyR, or in patients not achieving a CHR an increasing WBC despite appropriate therapeutic management. The protocol specified that the progression analysis would compare the intent to treat (ITT) population: patients randomized to receive Gleevec were compared with patients randomized to receive IFN. Patients that crossed over prior to progression were not censored at the time of cross-over, and events that occurred in these patients following cross-over were attributed to the original randomized treatment. The estimated rate of progression-free survival at 84 months in the ITT population was 81.2 % [95% CI: 78, 85] in the Gleevec arm and 60.6 % [56, 65] in the IFN arm (p<0.0001, log-rank test), (Figure 1). With 7 years follow up there were 93 (16.8%) progression events in the Gleevec arm: 37(6.7%) progression to AP/BC, 31(5.6%) loss of MCyR, 15 (2.7%) loss of CHR or increase in WBC and 10 (1.8%) CML unrelated deaths. In contrast, there were 165 (29.8%) events in the IFN+Ara-C arm of which 130 occurred during first-line treatment with IFN-Ara-C. The estimated rate of patients free of progression to accelerated phase (AP) or blast crisis (BC) at 84 months was 92.5%[90, 95] in the Gleevec arm compared to the 85.1%, [82, 89] (p≤0.001) in the IFN arm, (Figure 2). The annual rates of any progression events have decreased with time on therapy. The probability of remaining progression free at 60 months was 95% for patients who were in complete cytogenetic response (CCyR) with molecular response (≥3 log reduction in Bcr-Abl transcripts as measured by quantitative reverse transcriptase polymerase chain reaction) at 12 months, compared to 89% for patients in complete cytogenetic response but without a major molecular response and 70% in patients who were not in complete cytogenetic response at this time point (p<0.001).
- A total of 71 (12.8%) and 85 (15.4%) patients died in the Gleevec and IFN+Ara-C group, respectively. At 84 months the estimated overall survival is 86.4% (83, 90) vs. 83.3% (80, 87) in the randomized Gleevec and the IFN+Ara-C group, respectively (p=0.073 log-rank test). The hazard ratio is 0.750 with 95% CI 0.547-1.028. This time-to-event endpoint may be affected by the high crossover rate from IFN+Ara-C to Gleevec. Major cytogenetic response, hematologic response, evaluation of minimal residual disease (molecular response), time to accelerated phase or blast crisis and survival were main secondary endpoints. Response data are shown in Table 16. Complete hematologic response, major cytogenetic response and complete cytogenetic response were also statistically significantly higher in the Gleevec arm compared to the IFN + Ara-C arm (no cross-over data considered for evaluation of responses). Median time to CCyR in the 454 responders was 6 months (range 2-64 months, 25th to 75th percentiles=3 to 11 months) with 10% of responses seen only after 22 months of therapy).
*Molecular response was defined as follows:
- In the peripheral blood, after 12 months of therapy, reduction of ≥3 logarithms in the amount of bcr-abl transcripts (measured by real-time quantitative reverse transcriptase PCR assay) over a standardized baseline. Molecular response was only evaluated in a subset of patients who had a complete cytogenetic response by 12 months or later (N=333). The molecular response rate in patients who had a complete cytogenetic response in the Gleevec arm was 59% at 12 months and 72% at 24 months.
- Physical, functional, and treatment-specific biologic response modifier scales from the FACT-BRM (Functional Assessment of Cancer Therapy - Biologic Response Modifier) instrument were used to assess patient-reported general effects of interferon toxicity in 1,067 patients with CML in chronic phase. After one month of therapy to six months of therapy, there was a 13%-21% decrease in median index from baseline in patients treated with IFN, consistent with increased symptoms of IFN toxicity. There was no apparent change from baseline in median index for patients treated with Gleevec.
- Late Chronic Phase CML and Advanced Stage CML:
- Three international, open-label, single-arm phase 2 studies were conducted to determine the safety and efficacy of Gleevec in patients with Ph+ CML: 1) in the chronic phase after failure of IFN therapy, 2) in accelerated phase disease, or 3) in myeloid blast crisis. About 45% of patients were women and 6% were Black. In clinical studies 38%-40% of patients were ≥60 years of age and 10%-12% of patients were ≥70 years of age.
- Chronic Phase, Prior Interferon-Alpha Treatment:
- 532 patients were treated at a starting dose of 400 mg; dose escalation to 600 mg was allowed. The patients were distributed in three main categories according to their response to prior interferon: failure to achieve (within 6 months), or loss of a complete hematologic response (29%), failure to achieve (within 1 year) or loss of a major cytogenetic response (35%), or intolerance to interferon (36%). Patients had received a median of 14 months of prior IFN therapy at doses ≥25 x 106 IU/week and were all in late chronic phase, with a median time from diagnosis of 32 months. Effectiveness was evaluated on the basis of the rate of hematologic response and by bone marrow exams to assess the rate of major cytogenetic response (up to 35% Ph+ metaphases) or complete cytogenetic response (0% Ph+ metaphases). Median duration of treatment was 29 months with 81% of patients treated for ≥24 months (maximum = 31.5 months). Efficacy results are reported in Table 16. Confirmed major cytogenetic response rates were higher in patients with IFN intolerance (66%) and cytogenetic failure (64%), than in patients with hematologic failure (47%). Hematologic response was achieved in 98% of patients with cytogenetic failure, 94% of patients with hematologic failure, and 92% of IFN-intolerant patients.
- Accelerated Phase:
- 235 patients with accelerated phase disease were enrolled. These patients met one or more of the following criteria: ≥15%-<30% blasts in PB or BM; ≥30% blasts + promyelocytes in PB or BM; ≥20% basophils in PB; and <100 x 109/L platelets. The first 77 patients were started at 400 mg, with the remaining 158 patients starting at 600 mg.
- Effectiveness was evaluated primarily on the basis of the rate of hematologic response, reported as either complete hematologic response, no evidence of leukemia (i.e., clearance of blasts from the marrow and the blood, but without a full peripheral blood recovery as for complete responses), or return to chronic phase CML. Cytogenetic responses were also evaluated. Median duration of treatment was 18 months with 45% of patients treated for ≥24 months (maximum=35 months). Efficacy results are reported in Table 17. Response rates in accelerated phase CML were higher for the 600 mg dose group than for the 400 mg group: hematologic response (75% vs. 64%), confirmed and unconfirmed major cytogenetic response (31% vs. 19%).
- Myeloid Blast Crisis:
- 260 patients with myeloid blast crisis were enrolled. These patients had ≥30% blasts in PB or BM and/or extramedullary involvement other than spleen or liver; 95 (37%) had received prior chemotherapy for treatment of either accelerated phase or blast crisis (“pretreated patients”) whereas 165 (63%) had not (“untreated patients”). The first 37 patients were started at 400 mg; the remaining 223 patients were started at 600 mg.
- Effectiveness was evaluated primarily on the basis of rate of hematologic response, reported as either complete hematologic response, no evidence of leukemia, or return to chronic phase CML using the same criteria as for the study in accelerated phase. Cytogenetic responses were also assessed. Median duration of treatment was 4 months with 21% of patients treated for ≥12 months and 10% for ≥24 months (maximum=35 months). Efficacy results are reported in Table 17. The hematologic response rate was higher in untreated patients than in treated patients (36% vs. 22%, respectively) and in the group receiving an initial dose of 600 mg rather than 400 mg (33% vs. 16%). The confirmed and unconfirmed major cytogenetic response rate was also higher for the 600 mg dose group than for the 400 mg dose group (17% vs. 8%).
- The median time to hematologic response was 1 month. In late chronic phase CML, with a median time from diagnosis of 32 months, an estimated 87.8% of patients who achieved MCyR maintained their response 2 years after achieving their initial response. After 2 years of treatment, an estimated 85.4% of patients were free of progression to AP or BC, and estimated overall survival was 90.8% [88.3, 93.2]. In accelerated phase, median duration of hematologic response was 28.8 months for patients with an initial dose of 600 mg (16.5 months for 400 mg). An estimated 63.8% of patients who achieved MCyR were still in response 2 years after achieving initial response. The median survival was 20.9 [13.1, 34.4] months for the 400 mg group and was not yet reached for the 600 mg group (p=0.0097). An estimated 46.2% [34.7, 57.7] vs. 65.8% [58.4, 73.3] of patients were still alive after 2 years of treatment in the 400 mg vs. 600 mg dose groups, respectively. In blast crisis, the estimated median duration of hematologic response is 10 months. An estimated 27.2% [16.8, 37.7] of hematologic responders maintained their response 2 years after achieving their initial response. Median survival was 6.9 [5.8, 8.6] months, and an estimated 18.3% [13.4, 23.3] of all patients with blast crisis were alive 2 years after start of study.
- Efficacy results were similar in men and women and in patients younger and older than age 65. Responses were seen in Black patients, but there were too few Black patients to allow a quantitative comparison.
Pediatric CML
- A total of 51 pediatric patients with newly diagnosed and untreated CML in chronic phase were enrolled in an open-label, multicenter, single arm phase 2 trial. Patients were treated with Gleevec 340 mg/m2/day, with no interruptions in the absence of dose limiting toxicity. Complete hematologic response (CHR) was observed in 78% of patients after 8 weeks of therapy. The complete cytogenetic response rate (CCyR) was 65%, comparable to the results observed in adults. Additionally, partial cytogenetic response (PCyR) was observed in 16%. The majority of patients who achieved a CCyR developed the CCyR between months 3 and 10 with a median time to response based on the Kaplan-Meier estimate of 6.74 months. Patients were allowed to be removed from protocol therapy to undergo alternative therapy including hematopoietic stem cell transplantation. Thirty one children received stem cell transplantation. Of the 31 children, 5 were transplanted after disease progression on study and 1 withdrew from study during first week treatment and received transplant approximately 4 months after withdrawal. Twenty five children withdrew from protocol therapy to undergo stem cell transplant after receiving a median of 9 twenty-eight day courses (range 4 to 24). Of the 25 patients 13 (52%) had CCyR and 5 (20%) had PCyR at the end of protocol therapy.
- One open-label, single-arm study enrolled 14 pediatric patients with Ph+ chronic phase CML recurrent after stem cell transplant or resistant to interferon-alpha therapy. These patients had not previously received Gleevec and ranged in age from 3-20 years old; 3 were 3-11 years old, 9 were 12-18 years old, and 2 were >18 years old. Patients were treated at doses of 260 mg/m2/day (n=3), 340 mg/m2/day (n=4), 440 mg/m2/day (n=5) and 570 mg/m2/day (n=2). In the 13 patients for whom cytogenetic data are available, 4 achieved a major cytogenetic response, 7 achieved a complete cytogenetic response, and 2 had a minimal cytogenetic response.
- In a second study, 2 of 3 patients with Ph+ chronic phase CML resistant to interferon-alpha therapy achieved a complete cytogenetic response at doses of 242 and 257 mg/m2/day.
Acute Lymphoblastic Leukemia
- A total of 48 Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) patients with relapsed/refractory disease were studied, 43 of whom received the recommended Gleevec dose of 600 mg/day. In addition 2 patients with relapsed/refractory Ph+ ALL received Gleevec 600 mg/day in a phase 1 study.
- Confirmed and unconfirmed hematologic and cytogenetic response rates for the 43 relapsed/refractory Ph+ALL phase 2 study patients and for the 2 phase 1 patients are shown in Table 18. The median duration of hematologic response was 3.4 months and the median duration of MCyR was 2.3 months.
Pediatric ALL
- Pediatric and young adult patients with very high risk ALL, defined as those with an expected 5 year event-free survival (EFS) less than 45%, were enrolled after induction therapy on a multicenter, non-randomized cooperative group pilot protocol.
- The safety and effectiveness of Gleevec (340 mg/m2/day) in combination with intensive chemotherapy was evaluated in a subgroup of patients with Ph+ ALL. The protocol included intensive chemotherapy and hematopoietic stem cell transplant after 2 courses of chemotherapy for patients with an appropriate HLA-matched family donor. There were 92 eligible patients with Ph+ ALL enrolled. The median age was 9.5 years (1 to 21 years), 64% were male, 75% were white, 9% were Asian/Pacific Islander, and 5% were black. In 5 successive cohorts of patients, Gleevec exposure was systematically increased by earlier introduction and prolonged duration. Cohort 1 received the lowest intensity and cohort 5 received the highest intensity of Gleevec exposure.
- There were 50 patients with Ph+ ALL assigned to cohort 5 all of whom received Gleevec plus chemotherapy; 30 were treated exclusively with chemotherapy and Gleevec and 20 received chemotherapy plus Gleevec and then underwent hematopoietic stem cell transplant, followed by further Gleevec treatment. Patients in cohort 5 treated with chemotherapy received continuous daily exposure to Gleevec beginning in the first course of post induction chemotherapy continuing through maintenance cycles 1 through 4 chemotherapy. During maintenance cycles 5 through 12 Gleevec was administered 28 days out of the 56 day cycle. Patients who underwent hematopoietic stem cell transplant received 42 days of Gleevec prior to HSCT, and 28 weeks (196 days) of Gleevec after the immediate post transplant period. The estimated 4-year EFS of patients in cohort 5 was 70% (95% CI: 54, 81). The median follow-up time for EFS at data cutoff in cohort 5 was 40.5 months.
Myelodysplastic/Myeloproliferative Diseases
- An open label, multicenter, phase 2 clinical trial was conducted testing Gleevec in diverse populations of patients suffering from life-threatening diseases associated with Abl, Kit or PDGFR protein tyrosine kinases. This study included 7 patients with MDS/MPD. These patients were treated with Gleevec 400 mg daily. The ages of the enrolled patients ranged from 20 to 86 years. A further 24 patients with MDS/MPD aged 2 to 79 years were reported in 12 published case reports and a clinical study. These patients also received Gleevec at a dose of 400 mg daily with the exception of three patients who received lower doses. Of the total population of 31 patients treated for MDS/MPD, 14 (45%) achieved a complete hematological response and 12 (39%) a major cytogenetic response (including 10 with a complete cytogenetic response). Sixteen patients had a translocation, involving chromosome 5q33 or 4q12, resulting in a PDGFR gene re-arrangement. All of these patients responded hematologically (13 completely). Cytogenetic response was evaluated in 12 out of 14 patients, all of whom responded (10 patients completely). Only 1(7%) out of the 14 patients without a translocation associated with PDGFR gene re-arrangement achieved a complete hematological response and none achieved a major cytogenetic response. A further patient with a PDGFR gene re-arrangement in molecular relapse after bone marrow transplant responded molecularly. Median duration of therapy was 12.9 months (0.8-26.7) in the 7 patients treated within the phase 2 study and ranged between 1 week and more than 18 months in responding patients in the published literature. Results are provided in Table 19. Response durations of phase 2 study patients ranged from 141+ days to 457+ days.
Aggressive Systemic Mastocytosis
- One open-label, multicenter, phase 2 study was conducted testing Gleevec in diverse populations of patients with life-threatening diseases associated with Abl, Kit or PDGFR protein tyrosine kinases. This study included 5 patients with aggressive systemic mastocytosis (ASM) treated with 100 mg to 400 mg of Gleevec daily. These 5 patients ranged from 49 to 74 years of age. In addition to these 5 patients, 10 published case reports and case series describe the use of Gleevec in 23 additional patients with ASM aged 26 to 85 years who also received 100 mg to 400 mg of Gleevec daily.
- Cytogenetic abnormalities were evaluated in 20 of the 28 ASM patients treated with Gleevec from the published reports and in the phase 2 study. Seven of these 20 patients had the FIP1L1-PDGFRα fusion kinase (or CHIC2 deletion). Patients with this cytogenetic abnormality were predominantly males and had eosinophilia associated with their systemic mast cell disease. Two patients had a Kit mutation in the juxtamembrane region (one Phe522Cys and one K509I) and four patients had a D816V c-Kit mutation (not considered sensitive to Gleevec), one with concomitant CML.
- Of the 28 patients treated for ASM, 8 (29%) achieved a complete hematologic response and 9 (32%) a partial hematologic response (61% overall response rate). Median duration of Gleevec therapy for the 5 ASM patients in the phase 2 study was 13 months (range 1.4-22.3 months) and between 1 month and more than 30 months in the responding patients described in the published medical literature. A summary of the response rates to Gleevec in ASM is provided in Table 20. Response durations of literature patients ranged from 1+ to 30+ months.
|howSupplied=
|fdaPatientInfo=
There is limited information regarding Patient Counseling Information of Imatinib in the drug label.
|alcohol=
- Alcohol-Imatinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=
- ®[1]
|lookAlike=
- A® — B®[2]
|drugShortage=
}}
{{#subobject:
|Page Name=Imatinib |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Imatinib |Label Name=Imatinib11.png
}}
{{#subobject:
|Label Page=Imatinib |Label Name=Imatinib11.png
}}
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)