IDH2
| Isocitrate dehydrogenase 2 (NADP+), mitochondrial | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
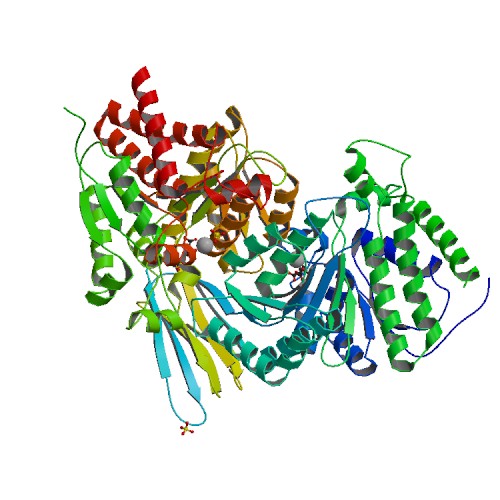 PDB rendering based on 1lwd. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | IDH2 ; IDH; IDP; ICD-M; IDHM; mNADP-IDH | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 37590 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
 | |||||||||||||
 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Isocitrate dehydrogenase 2 (NADP+), mitochondrial, also known as IDH2, is a human gene.[1]
Isocitrate dehydrogenases are enzymes that catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate. These enzymes belong to two distinct subclasses, one of which utilizes NAD(+) as the electron acceptor and the other NADP(+). Five isocitrate dehydrogenases have been reported: three NAD(+)-dependent isocitrate dehydrogenases, which localize to the mitochondrial matrix, and two NADP(+)-dependent isocitrate dehydrogenases, one of which is mitochondrial and the other predominantly cytosolic. Each NADP(+)-dependent isozyme is a homodimer. The protein encoded by the IDH2 gene is the NADP(+)-dependent isocitrate dehydrogenase found in the mitochondria. It plays a role in intermediary metabolism and energy production. This protein may tightly associate or interact with the pyruvate dehydrogenase complex.[1]
References
Further reading
- Bruns GA, Eisenman RE, Gerald PS (1977). "Human mitochondrial NADP-dependent isocitrate dehydrogenase in man-mouse somatic cell hybrids". Cytogenet. Cell Genet. 17 (4): 200–11. PMID 11969.
- Shimizu N, Giles RE, Kucherlapati RS; et al. (1978). "Somatic cell genetic assignment of the human gene for mitochondrial NADP-linked isocitrate dehydrogenase to the long arm of chromosome 15". Somatic Cell Genet. 3 (1): 47–60. PMID 564083.
- Champion MJ, Brown JA, Shows TB (1979). "Assignment of cytoplasmic alpha-mannosidase (MANA) and confirmation of mitochondrial isocitrate dehydrogenase (IDHM) to the q11 leads to qter region of chromosome 15 in man". Cytogenet. Cell Genet. 22 (1–6): 498–502. PMID 752528.
- Grzeschik KH (1976). "Assignment of a gene for human mitochondrial isocitrate dehydrogenase (ICD-M, EC 1.1.1.41) to chromosome 15". Hum. Genet. 34 (1): 23–8. PMID 965003.
- Klimek J, Boguslawski W, Tialowska B, Zelewski L (1976). "Regulation of progesterone biosynthesis in human placental mitochondria by Krebs cycle metabolites". Acta Biochim. Pol. 23 (2–3): 185–92. PMID 970033.
- Chamberlain KG, Penington DG (1988). "Monoamine oxidase and other mitochondrial enzymes in density subpopulations of human platelets". Thromb. Haemost. 59 (1): 29–33. PMID 3363531.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. PMID 8125298.
- Luo H, Shan X, Wu J (1996). "Expression of human mitochondrial NADP-dependent isocitrate dehydrogenase during lymphocyte activation". J. Cell. Biochem. 60 (4): 495–507. doi:10.1002/(SICI)1097-4644(19960315)60:4%3C495::AID-JCB6%3E3.0.CO;2-N. PMID 8707889.
- Oh IU, Inazawa J, Kim YO; et al. (1997). "Assignment of the human mitochondrial NADP(+)-specific isocitrate dehydrogenase (IDH2) gene to 15q26.1 by in situ hybridization". Genomics. 38 (1): 104–6. doi:10.1006/geno.1996.0602. PMID 8954790.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K; et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. PMID 9373149.
- Strausberg RL, Feingold EA, Grouse LH; et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMID 12477932.
- Gevaert K, Goethals M, Martens L; et al. (2004). "Exploring proteomes and analyzing protein processing by mass spectrometric identification of sorted N-terminal peptides". Nat. Biotechnol. 21 (5): 566–9. doi:10.1038/nbt810. PMID 12665801.
- Gerhard DS, Wagner L, Feingold EA; et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMID 15489334.
- Foster LJ, Rudich A, Talior I; et al. (2006). "Insulin-dependent interactions of proteins with GLUT4 revealed through stable isotope labeling by amino acids in cell culture (SILAC)". J. Proteome Res. 5 (1): 64–75. doi:10.1021/pr0502626. PMID 16396496.
- Kil IS, Kim SY, Lee SJ, Park JW (2007). "Small interfering RNA-mediated silencing of mitochondrial NADP+-dependent isocitrate dehydrogenase enhances the sensitivity of HeLa cells toward tumor necrosis factor-alpha and anticancer drugs". Free Radic. Biol. Med. 43 (8): 1197–207. doi:10.1016/j.freeradbiomed.2007.07.009. PMID 17854715.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |