Hypromellose: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
Rabin Bista (talk | contribs) No edit summary |
||
| (12 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{RB}} | |authorTag={{RB}} | ||
|OTC=Yes | |||
|genericName=Hypromellose | |||
|aOrAn=a | |aOrAn=a | ||
| | |drugClass=ophthalmic solution | ||
|adverseReactions= | |indicationType=treatment | ||
|blackBoxWarningTitle=<span style="color:#FF0000;"> | |indication=[[dryness of the eye]] and prevent further irritation | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"> | |adverseReactions=[[eye pain]], [[changes in vision]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult=*Relieves [[dryness of the eye]]. | |||
*Temporarily relieves discomfort due to minor irritations of the eye or from exposure to wind and sun. | |||
*As a protectant against further irritation. | |||
====Dosage==== | |||
|fdaLIADAdult= | *Put 1 or 2 drops in the affected eye(s) as needed. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
* | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
* | |||
==== | |||
* | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|warnings=* For external use only. | |||
* Do not use if sealed bottle tip is broken or punctured. | |||
* If solution changes color or becomes cloudy, do not use. | |||
====When using this product==== | |||
* To avoid contamination, do not touch tip of container to any surface. | |||
* Replace cap after using. | |||
====Stop use and ask a physician==== | |||
* If you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor. | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
| | |||
==== | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials= | |clinicalTrials=* [[eye pain]] | ||
* [[changes in vision]] | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInPregnancyAUS= | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | ||
| Line 264: | Line 57: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* Ophthalmic solution | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
|overdose=There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
|overdose= | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox= | |drugBox={{Chembox2 | ||
| | | verifiedrevid = 451585076 | ||
| ImageFile = Hypromellose Wiki Structure.png | |||
| ImageSize = 200px | |||
| | | IUPACName = | ||
| OtherNames = Hydroxypropyl methylcellulose; hydroxypropyl methyl cellulose; HPMC; E464 | |||
| Section1 = {{Chembox Identifiers | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 21241863 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 36SFW2JZ0W | |||
| InChI = 1/C36H70O19.C20H38O11/c1-19(37)9-45-17-27-29(47-11-21(3)39)31(48-12-22(4)40)34(51-15-25(7)43)36(54-27)55-30-28(18-46-10-20(2)38)53-35(52-16-26(8)44)33(50-14-24(6)42)32(30)49-13-23(5)41;1-21-9-11-13(23-3)15(24-4)18(27-7)20(30-11)31-14-12(10-22-2)29-19(28-8)17(26-6)16(14)25-5/h19-44H,9-18H2,1-8H3;11-20H,9-10H2,1-8H3/t19?,20?,21?,22?,23?,24?,25?,26?,27-,28-,29-,30-,31+,32+,33-,34-,35-,36+;11-,12-,13-,14-,15+,16+,17-,18-,19-,20+/m11/s1 | |||
| InChIKey = PUSNGFYSTWMJSK-GSZQVNRLBE | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C36H70O19.C20H38O11/c1-19(37)9-45-17-27-29(47-11-21(3)39)31(48-12-22(4)40)34(51-15-25(7)43)36(54-27)55-30-28(18-46-10-20(2)38)53-35(52-16-26(8)44)33(50-14-24(6)42)32(30)49-13-23(5)41;1-21-9-11-13(23-3)15(24-4)18(27-7)20(30-11)31-14-12(10-22-2)29-19(28-8)17(26-6)16(14)25-5/h19-44H,9-18H2,1-8H3;11-20H,9-10H2,1-8H3/t19?,20?,21?,22?,23?,24?,25?,26?,27-,28-,29-,30-,31+,32+,33-,34-,35-,36+;11-,12-,13-,14-,15+,16+,17-,18-,19-,20+/m11/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = PUSNGFYSTWMJSK-GSZQVNRLSA-N | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CASNo = 9004-65-3 | |||
| PubChem = | |||
| ATCCode_prefix = S01 | |||
| ATCCode_suffix = KA02 | |||
| SMILES = }} | |||
| Section2 = {{Chembox Properties | |||
| Formula = variable | |||
| MolarMass = variable | |||
| Appearance = | |||
| Density = | |||
| MeltingPt = | |||
| BoilingPt = | |||
| Solubility = }} | |||
| Section3 = {{Chembox Hazards | |||
| MainHazards = | |||
| FlashPt = | |||
| Autoignition = }} | |||
}} | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
| Line 313: | Line 113: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
| | |storage=* Store at room temperature 15°-30°C (59°-86°F). | ||
|packLabel= | * Do not freeze. | ||
|fdaPatientInfo= | * Keep box for complete information. | ||
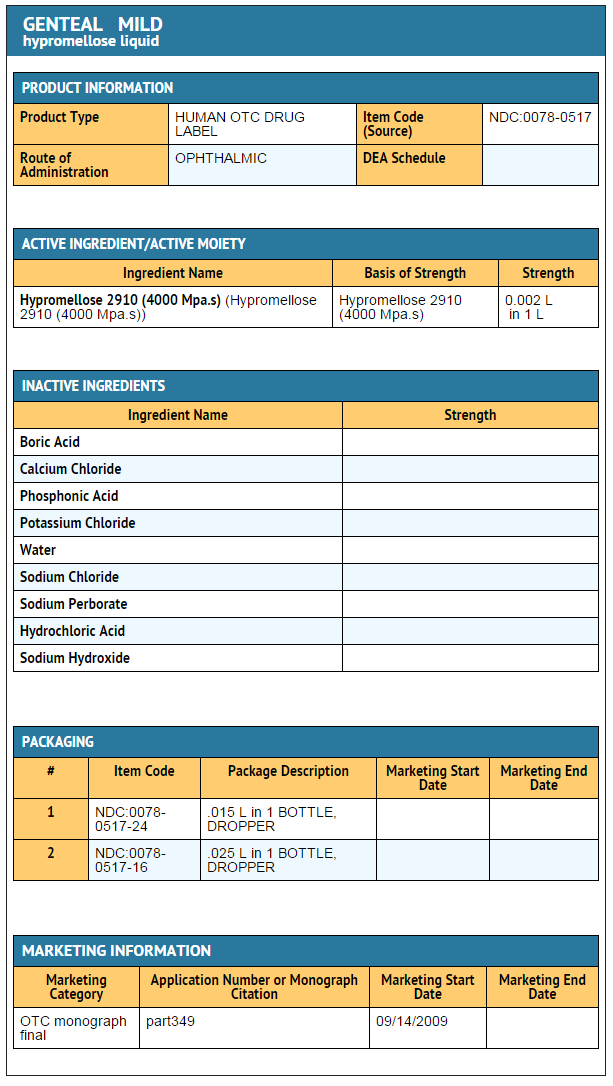
|packLabel=====Principal display panel==== | |||
: [[File:Hypromellose Principal Display panel.png|none|600px]] | |||
====Ingredients and Appearance==== | |||
: [[File:Hypromellose Ingredients and Appearance.png|none|600px]] | |||
|fdaPatientInfo=* When using this product do not touch tip of container to any surface. Replace cap after using. | |||
* Stop use and ask a doctor if you experience any of the following: | |||
:* eye pain | |||
:* changes in vision | |||
:* continued redness or irritation of the eye | |||
:* condition worsens or persists for more than 72 hours | |||
* If swallowed, get medical help or contact a Poison Control Center right away. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* GenTeal®<ref>{{Cite web | title = Hypromellose | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3c4ea323-2407-4f9d-b44f-eebf127924ba }}</ref> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 15:28, 24 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Hypromellose is a ophthalmic solution that is FDA approved for the treatment of dryness of the eye and prevent further irritation. Common adverse reactions include eye pain, changes in vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Relieves dryness of the eye.
- Temporarily relieves discomfort due to minor irritations of the eye or from exposure to wind and sun.
- As a protectant against further irritation.
Dosage
- Put 1 or 2 drops in the affected eye(s) as needed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hypromellose in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hypromellose in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Hypromellose in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hypromellose in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hypromellose in pediatric patients.
Contraindications
There is limited information regarding Hypromellose Contraindications in the drug label.
Warnings
- For external use only.
- Do not use if sealed bottle tip is broken or punctured.
- If solution changes color or becomes cloudy, do not use.
When using this product
- To avoid contamination, do not touch tip of container to any surface.
- Replace cap after using.
Stop use and ask a physician
- If you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor.
Adverse Reactions
Clinical Trials Experience
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Hypromellose in the drug label.
Drug Interactions
There is limited information regarding Hypromellose Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Hypromellose in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hypromellose in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Hypromellose during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Hypromellose with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Hypromellose with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Hypromellose with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Hypromellose with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hypromellose with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Hypromellose in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Hypromellose in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hypromellose in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hypromellose in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic solution
Monitoring
There is limited information regarding Monitoring of Hypromellose in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Hypromellose in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Hypromellose in the drug label.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox E numberTemplate:Chembox Supplement| Template:Chembox header2 | Hypromellose | |
|---|---|
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| UNII | |
| |
| Properties | |
| variable | |
| Molar mass | variable |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
There is limited information regarding Hypromellose Mechanism of Action in the drug label.
Structure
There is limited information regarding Hypromellose Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Hypromellose in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Hypromellose in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Hypromellose in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Hypromellose in the drug label.
How Supplied
There is limited information regarding Hypromellose How Supplied in the drug label.
Storage
- Store at room temperature 15°-30°C (59°-86°F).
- Do not freeze.
- Keep box for complete information.
Images
Drug Images
{{#ask: Page Name::Hypromellose |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Principal display panel
Ingredients and Appearance
{{#ask: Label Page::Hypromellose |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- When using this product do not touch tip of container to any surface. Replace cap after using.
- Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
- If swallowed, get medical help or contact a Poison Control Center right away.
Precautions with Alcohol
- Alcohol-Hypromellose interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GenTeal®[1]
Look-Alike Drug Names
There is limited information regarding Hypromellose Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.