Hepatitis C/Lab Tests
Page has default form::MedicalTest {{#meta: itemprop="medicalWebPageAudiences" content="patient"}}{{#meta: itemprop="medicalWebPageSpecialities" content="cardiology"}}{{#meta: itemprop="medicalWebPageInfoTypes" content="symptoms,diagnosis,treatment,causes,prognosis,complications"}}
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Varun Kumar, M.B.B.S. [2]; Assistant Editor-In-Chief: Nina Axiotakis [3]
Overview
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C/Lab Tests On the Web |
|
American Roentgen Ray Society Images of Hepatitis C/Lab Tests |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4] ; Associate Editor(s)-In-Chief: Javaria Anwer M.D.[5]
Overview
The laboratory diagnosis of hepatitis C infection is first made by documenting positive serologies (anti-HCV antibodies), followed by HCV RNA quantification by PCR or NAT to determine the viral load and to differentiate chronic infection from remission.
Serologies and HCV RNA
The diagnosis of HCV is rarely made during the acute phase given that the majority of individuals infected are asymptomatic during this phase of the disease. Chronic hepatitis C may be suspected on the basis of the medical history (particularly if there is the history of IV or intranasal drug use), a history of piercings or tattoos, unexplained symptoms, or abnormal levels of liver function tests found during routine blood testing.
Hepatitis C testing begins with serological blood tests used to detect antibodies against HCV in the majority of cases. Overall, anti-HCV antibody tests have a strong positive predictive value for exposure to the hepatitis C virus, but may miss patients who have not yet had seroconversion.[1][2] As anti-HCV antibodies indicate exposure to the virus, but cannot determine active infection, all patients with positive anti-HCV antibody tests must undergo HCV RNA quantification by nucleic acid amplification (NAT) via polymerase chain reaction (PCR) to determine the viral load. The HCV viral load is an important factor in determining active disease and the probability of response to therapy. It is not associated with disease severity or the likelihood of disease progression.
Rarely, HCV RNA quantification is performed without prior anti-HCV antibody testing. This is only indicated in patients who have a known past history of cleared HCV infection with previous seroconversion and in immunocompromised patients.
Among individuals with confirmed HCV infection, genotype testing is recommended. HCV genotype testing is used tailor therapeutic regimen.[3]
Laboratory Tests
- HCV Enzyme-linked immunosorbent assay (ELISA)
- Positive within 4-10 weeks after infection
- False negatives can occur with HIV infection, chronic renal failure, and cryoglobulinemia
- HCV RNA
- PCR highly sensitive for confirming viremia
- Predicts response to therapy but not risk of progression
A single positive PCR test indicates infection with HCV. Negative tests usually do not require re-testing, except in cases with high clinical suspicion.
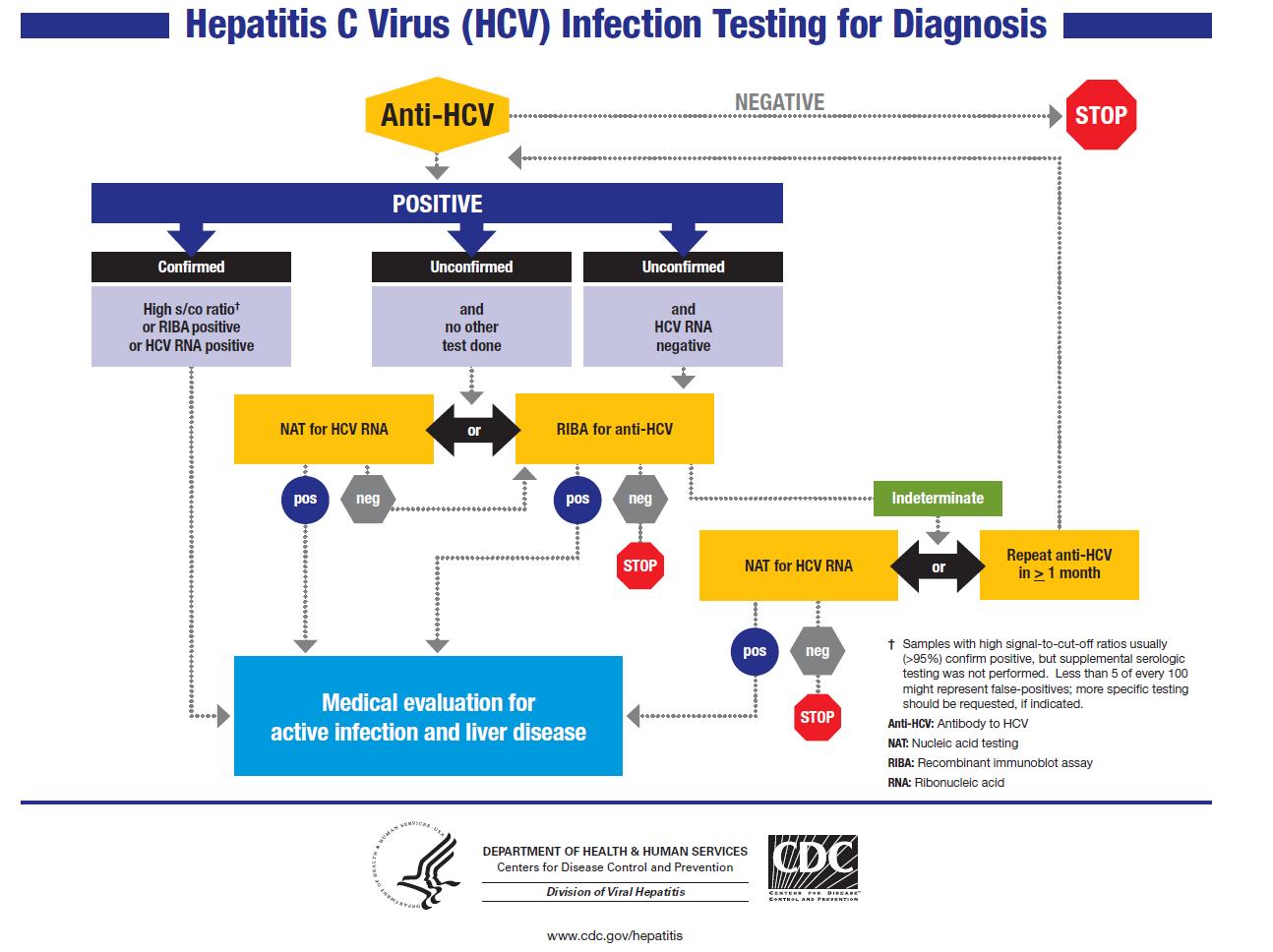
Follow-up Testing for Healthcare Personnel Exposed to HCV-positive Blood
- For the source, perform baseline serological testing using anti-HCV antibodies.
- For the person exposed to an HCV-positive source, perform baseline and follow-up testing, including
- baseline testing for anti-HCV and ALT activity, and
- follow-up testing for anti-HCV and ALT activity at approximately 4–6 months after exposure. If earlier diagnosis of HCV infection is desired, testing for HCV RNA may be performed at 4–6 weeks after exposure
- Confirmation by supplemental HCV RNA testing for all positive anti-HCV results
References
- ↑ AASLD/IDSA/IAS–USA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed July 27, 2014.
- ↑ Cacoub P, Desbois AC, Comarmond C, Saadoun D (November 2018). "Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis". Gut. 67 (11): 2025–2034. doi:10.1136/gutjnl-2018-316234. PMID 29703790.
- ↑ World Health Organization (WHO) 2014. Guidelines for the screening, care and treatment of persons with hepatitis C infection.http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed online on July 24,2014.
Related Pages
- Signs Detected:
- Drug Interactions:
- Used To Diagnose:Used To Diagnose::Hepatitis C
- Uses Device: