Hepatitis B risk factors: Difference between revisions
| Line 12: | Line 12: | ||
* Sexual/household contacts of [[infected]] persons | * Sexual/household contacts of [[infected]] persons | ||
* Patients and employees in [[hemodialysis]] centers | * Patients and employees in [[hemodialysis]] centers | ||
* Injection drug users sharing | * Injection drug users sharing unsterilized needles | ||
* People sharing | * People sharing unsterilized medical or dental equipment | ||
* People providing or receiving acupuncture and/or tattooing with | * People providing or receiving acupuncture and/or tattooing with unsterilized medical devices | ||
* Persons living in regions or travelling to regions with endemic hepatitis B | * Persons living in regions or travelling to regions with [[endemic]] hepatitis B | ||
**Country of origin is the major risk factor for HBV infection (prevalence threshold of 2% or greater to define countries with high risk for HBV infection) | **Country of origin is the major risk factor for HBV infection (prevalence threshold of 2% or greater to define countries with high risk for HBV infection) | ||
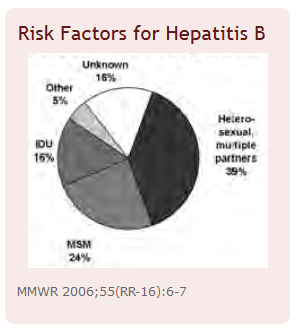
* Sexually active heterosexuals | * Sexually active heterosexuals | ||
Revision as of 19:02, 18 October 2016
|
Hepatitis B |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis B risk factors On the Web |
|
American Roentgen Ray Society Images of Hepatitis B risk factors |
|
Risk calculators and risk factors for Hepatitis B risk factors |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]; Sara Mehrsefat, M.D. [3]
Overview
Common risk factors in the development of HBV infection include sexual contact with infected individuals, sharing a household with a carrier, intravenous drug use, travel to endemic regions, perinatal transmission from infected mothers to infants, and certain occupations.
Risk Factors
Individuals who are at increased risk of hepatitis B infection include:[1][2]
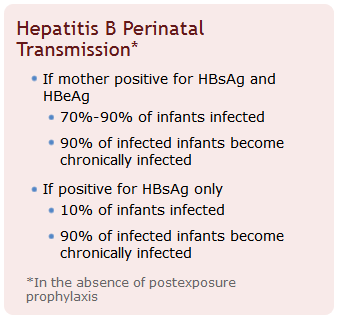
- Infants born to infected mothers
- Young children in day-care or residential settings with other children in endemic areas
- Sexual/household contacts of infected persons
- Patients and employees in hemodialysis centers
- Injection drug users sharing unsterilized needles
- People sharing unsterilized medical or dental equipment
- People providing or receiving acupuncture and/or tattooing with unsterilized medical devices
- Persons living in regions or travelling to regions with endemic hepatitis B
- Country of origin is the major risk factor for HBV infection (prevalence threshold of 2% or greater to define countries with high risk for HBV infection)
- Sexually active heterosexuals
- Lack of vaccination in infancy
- Men who have sex with men
- Hemophilia patients
- Travel to areas where hepatitis B is common
Frequent and routine exposure to blood or serum is the common denominator of healthcare occupational exposure.[3]
 |
 |
Hepatitis B Reactivation
Hepatitis B virus presents in all patients with infection. Patients who are either HBsAg positive or anti HBc positive are at the risk of heaptitis B reactivation. Hepatits B reactivation can occurs by following m Paitents are at risk for HBV reactivation in following conditions:[5][6][7][8][9][10]
- Receive immunosuppressive therapy
- Chemotherapy agents
- Tumor necrosis factor (TNF) inhibitor (Infliximab)
- Methotrexate (particularly following its withdrawal)
- Abatacept
- Ustekinumab
- Anti-CD 20 agents (Rituximab and Ofatumumab)
- High to moderate dose glucocorticoids
- Patients treated with direct-acting antivirals for hepatitis C
- Daklinza
- Epclusa
- Exviera
- Harvoni
- Olysio
- Sovaldi
- Viekira Pak/ Viekira Pak XR
- Technivie
- Zepatier
References
- ↑ World Health Organization. Department of Cummunicable Disease Surveillance and Response http://apps.who.int/iris/bitstream/10665/67746/1/WHO_CDS_CSR_LYO_2002.2_HEPATITIS_B.pdf
- ↑ US. Preventive Services Task Force. Screening for Hepatitis B infection. (2014) https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-b-virus-infection-screening-2014?ds=1&s=hepatitis%20b Accessed on October 4th, 2016
- ↑ "Hepatitis B" (PDF).
- ↑ 4.0 4.1 "Center for Disease Control and Prevention (CDC)".
- ↑ Lee YH, Bae SC, Song GG (2013). "Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy". Clin Exp Rheumatol. 31 (1): 118–21. PMID 23111095.
- ↑ Kim PS, Ho GY, Prete PE, Furst DE (2012). "Safety and efficacy of abatacept in eight rheumatoid arthritis patients with chronic hepatitis B." Arthritis Care Res (Hoboken). 64 (8): 1265–8. doi:10.1002/acr.21654. PMID 22392695.
- ↑ Sagnelli E, Manzillo G, Maio G, Pasquale G, Felaco FM, Filippini P; et al. (1980). "Serum levels of hepatitis B surface and core antigens during immunosuppressive treatment of HBsAg-positive chronic active hepatitis". Lancet. 2 (8191): 395–7. PMID 6105519.
- ↑ Nair PV, Tong MJ, Stevenson D, Roskamp D, Boone C (1985). "Effects of short-term, high-dose prednisone treatment of patients with HBsAg-positive chronic active hepatitis". Liver. 5 (1): 8–12. PMID 3884951.
- ↑ Europian Medicines Agency. reviews direct-acting antivirals for hepatitis C. (2016) http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Direct-acting_antivirals_for_hepatitis_C_20/Procedure_started/WC500203479.pdf
- ↑ U.S Food and Drug Adminestration. Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C http://www.fda.gov/downloads/Drugs/DrugSafety/UCM523499.pdf