Heart failure resident survival guide
For acute heart failure prevention click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mahmoud Sakr, M.D. [2]; Ayokunle Olubaniyi, M.B,B.S [3]
| Acute Heart Failure Resident Survival Guide Microchapters |
|---|
| Overview |
| Classification |
| Causes |
| FIRE |
| Diagnosis |
| Treatment |
| Do's |
| Don'ts |
Overview
Acute heart failure can occur in the setting of a new onset heart failure or worsening of an existing chronic heart failure (also known as acute decompensated heart failure, flash pulmonary edema, ADHF). ADHF presents with acute shortness of breath due to the development of pulmonary edema (the rapid accumulation of fluid in the lung). Other signs and symptoms of ADHF include hypotension with impaired and organ perfusion manifested by worsening renal function, altered mentation and cold clammy extremities. ADHF is associated with a poor prognosis if not treated aggressively. Like chronic heart failure therapy, the goal is to improve symptoms but unlike chronic therapy the other goals are to improve oxygenation and hemodynamic stability. The mainstays of the acute medical treatment in acute decompensated congestive heart failure include oxygen to improve hypoxia, diuresis to reduce both preload and intravascular volume and vasodilators to reduce afterload. Some of the mainstays of chronic heart failure therapy are not initiated acutely (ACE inhibitors, beta blockers and digoxin).
Classification
Based on the Severity of Congestive Heart Failure
The New York Heart Association (NYHA) assessment of heart failure severity is often used to guide treatment:
| NYHA classification |
Description |
|---|---|
| I | No limitation of physical activity. Ordinary physical activity does not cause symptoms of heart failure (HF) |
| II | Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in symptoms of HF |
| III | Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes symptoms of HF |
| IV | Unable to carry on any physical activity without symptoms of HF, or symptoms of HF at rest |
NYHA - New York Heart Association
Based on the Stage of Heart Failure
| ACCF/AHA Stages | Description |
|---|---|
| A | At high risk for heart failure (HF) but without structural heart disease or symptoms of HF |
| B | Structural heart disease but without signs or symptoms of HF |
| C | Structural heart disease with prior or current symptoms of HF |
| D | Refractory HF requiring specialized interventions |
ACCF - American College of Cardiology Foundation; AHA - American Heart Association
Based on Left Ventricular Ejection Fraction (LVEF)
- Heart failure with preserved ejection fraction (HFpEF) or diastolic heart failure: ejection fraction ≥ 50%
- Heart failure with reduced ejection fraction (HFrEF) or systolic heart failure: ejection fraction ≤ 40%
Causes
Life Threatening Causes
Acute decompensated heart failure is life threatening and should be treated as such irrespective of the underlying cause.
Common Causes
- Acute coronary syndrome
- Acute kidney injury
- Acute severe myocarditis
- Cardiac arrhythmias
- Cardiomyopathy
- Cardiotoxic agents - alcohol, cocaine
- Decompensation of an underlying chronic heart failure
- Hypertensive crisis
- Pulmonary embolus
- Systemic Inflammatory response syndrome
- Valvular heart disease
Click here for the complete list of causes.
FIRE: Focused Initial Rapid Evaluation
A Focused Initial Rapid Evaluation (FIRE) should be performed to identify patients in need of immediate intervention.
Boxes in red signify that an urgent management is needed.
Abbreviations:
MAP: Mean arterial pressure;
NYHA: New York Heart Association;
SBP: Systolic blood pressure
Identify cardinal findings that increase the pretest probability of acute heart failure ❑ Past medical history of heart failure | |||||||||||||||||||||||
Does the patient have any of the following findings that require urgent management? ❑ Hypotension (SBP < 90 mmHg or drop in MAP >30 mmHg) | |||||||||||||||||||||||
Yes | No | ||||||||||||||||||||||
Treat cardiogenic shock ❑ Admit to intensive care unit (ICU) or coronary care unit (CCU) for closer monitoring
❑ For SBP 85 - 100 mm Hg ❑ For SBP < 85 mm Hg ❑ Consider intra-aortic balloon pump, if hypotension persists | Does the patient have severe symptoms of heart failure? ❑ NYHA class III
❑ NYHA class IV
| ||||||||||||||||||||||
Yes | No | ||||||||||||||||||||||
Urgent treatment ❑ Diuretic therapy (click for details) every 3-5 mins as tolerated. Max of 400mcg/min OR nesiritide at 2 mcg/kg bolus; then 0.01 mcg/kg/minute continuous infusion. Max of 0.03 mcg/kg/minute | |||||||||||||||||||||||
Complete Diagnostic Approach
A complete diagnostic approach should be carried out after a focused initial rapid evaluation is conducted and following initiation of any urgent intervention.[2][3]
Abbreviations:
ARDS: Acute respiratory distress syndrome;
BNP: B-type natriuretic peptide;
BUN: Blood urea nitrogen;
CAD: Coronary artery disease;
CBC: Complete blood count;
CCB: Calcium channel blocker;
CT: Computed tomography;
CXR: Chest X-ray;
DM: Diabetes mellitus;
EKG: Electrocardiogram;
GDMT: Guideline-directed medical therapy;
HTN: Hypertension;
LVEF: Left ventricular ejection fraction;
LVH: Left ventricular hypertrophy;
MI: Myocardial infarction;
MRI: Magnetic resonance imaging;
NT-pro BNP: N-terminal pro-brain natriuretic peptide;
OCPs: Oral contraceptive pills;
PAWP: Pulmonary artery wedge pressure;
TSH: Thyroid stimulating hormone
Characterize the symptoms: Symptoms of fluid accumulation
❑ Paroxysmal nocturnal dyspnea Obtain a detailed history:
❑ Medication history
❑ Family history
| |||||||||||||||||||||||||||||||||
Examine the patient: General appearance: Vitals: ❑ Pulse
❑ Pulse oximetry assure sat is > 90% Weight: Skin Respiratory examination Cardiovascular examination
Abdominal examination Extremity examination Neurological examination | |||||||||||||||||||||||||||||||||
Order tests: Routine (Class I, level of evidence C)
❑ BNP or NT-pro BNP (if diagnosis is uncertain)
❑ Chest X-ray (Class I, level of evidence C)
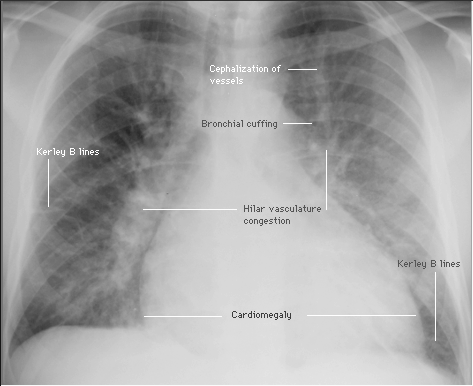 ❑ EKG
❑ 2-D echocardiography with Doppler
❑ Radionuclide ventriculography or MRI
❑ Coronary angiography (in settings of ischemia) Order additional tests to rule out other etiologies: | |||||||||||||||||||||||||||||||||
Consider alternative diagnoses:
| |||||||||||||||||||||||||||||||||
Assess the stage of heart failure using the ACCF/AHA staging system to guide chronic therapy
| |||||||||||||||||||||||||||||||||
Stage C ❑ Patients with structural heart disease
❑ Signs or symptoms of heart failure | |||||||||||||||||||||||||||||||||
Treatment
The treatment of acute heart failure is largely dependent on whether the patient has a preserved ejection fraction (diastolic heart failure) or reduced ejection fraction (systolic heart failure)
Initial stabilization: ❑ Assess the airway
❑ Ensure continuous cardiac monitoring | |||||||||||||||||||||||||||||||||
Consider admission if the following is present:[6] ❑ Hypotension and/or cardiogenic shock | |||||||||||||||||||||||||||||||||
Treat precipitating causes/co-morbidities Click for detailed management ❑ Atrial fibrillation
Avoid the use of drugs with negative inotropic effects such as beta blockers and non-dihydropyridine calcium channel blockers e.g., verapamil in the treatment of acute decompensated systolic heart failure
Note: Unfractionated heparin should be administered before cardioversion | |||||||||||||||||||||||||||||||||
Assess hemodynamic and volume status[7] ❑ Congestion at rest (dry vs. wet) "Cold" suggested by narrow pulse pressure, cool extremities, hypotension | |||||||||||||||||||||||||||||||||
| Classify the patient based on the left ventricular ejection fraction | |||||||||||||||||||||||||||||||||
| Diastolic heart failure LVEF ≥ 50% | Systolic heart failure LVEF ≤ 40% | ||||||||||||||||||||||||||||||||
Treatment ❑ Rate control - to prolong left ventricular filling time
❑ Diuretic therapy to reduce volume overload (click for details)
| Treatment Consider the following:
❑ Beta blockers[8]
❑ Aldosterone antagonists
| ||||||||||||||||||||||||||||||||
Indications for implantable cardioverter defibrillator (ICD) ❑ As primary prevention of sudden cardiac death in:
Contraindications | |||||||||||||||||||||||||||||||||
Indications for cardiac transplantation ❑ Refractory cardiogenic shock | |||||||||||||||||||||||||||||||||
General measures ❑ Low sodium diet ❑ Daily serum electrolytes, urea & creatinine | |||||||||||||||||||||||||||||||||
Discharge and follow-Up ❑ Patient and family education
❑ Telephone follow-up call usually 3 days post discharge | |||||||||||||||||||||||||||||||||
Diuretic Therapy Details
| Evidence of volume overload | |||||||||||||||||||||
❑ Low sodium diet (<2 g daily)
Contraindications to IV Diuresis | |||||||||||||||||||||
| Symptomatic improvement? | |||||||||||||||||||||
| Yes | No | ||||||||||||||||||||
| Maintain current IV diuretic dose | Double IV diuretic dose and titrate according to patient's response or when the maximum dose is reached | ||||||||||||||||||||
| No symptomatic improvement | |||||||||||||||||||||
Add ❑ Another diuretic e.g., IV chlorothiazide or oral metolazone | Adjuvants to diuretics ❑ Low dose dopamine to preserve renal function and renal blood flow | ||||||||||||||||||||
| No symptomatic improvement (refractory edema) | |||||||||||||||||||||
| Ultrafiltration or dialysis | |||||||||||||||||||||
General measures ❑ Monitor BP, volume status, congestion ❑ Daily serum electrolytes, urea & creatinine ❑ DVT prophylaxis | |||||||||||||||||||||
Medications
| Drug Class | Drug | Daily dose | Maximum daily dose |
|---|---|---|---|
| Loop diuretics | Furosemide | 20 to 40 mg once or twice In HF patients on loop diuretic, the initial IV dose should be greater or equal to their chronic oral daily dose.[12] |
600 mg |
| Bumetanide | 0.5 to 1.0 mg once or twice | 10 mg | |
| Torsemide | 10 to 20 mg once | 200 mg | |
| Thiazide diuretics | Chlorothiazide | 250 to 500 mg once or twice | 1000 mg |
| Hydrochlorothiazide | 25 mg once or twice | 200 mg | |
| Metolazone | 2.5 mg once | 20 mg | |
| K+- sparing diuretic | Amiloride | 5 mg once | 20 mg |
| Spironolactone | 12.5 to 25.0 mg once | 50 mg | |
| Triamterene | 50 to 75 mg twice | 200 mg | |
| ACE inhibitors | Enalapril | 2.5 mg twice | 10 to 20 mg twice |
| Lisinopril | 2.5 to 5 mg once | 20 to 40 mg once | |
| Ramipril | 1.25 to 2.5 mg once | 10 mg once | |
| ARBs | Candesartan | 4 to 8 mg once | 32 mg once |
| Losartan | 25 to 50 mg once, 50 to 150 mg once | ||
| Valsartan | 20 to 40 mg twice | 160 mg twice | |
| Beta blockers | Bisoprolol | 1.25 mg once | 10 mg once |
| Carvedilol | 3.125 mg twice | 50 mg twice | |
| Metoprolol succinate | 12.5 to 25.0 mg once | 200 mg once | |
| Aldosterone antagonists | Spironolactone | 12.5 to 25.0 mg once | 25 mg once or twice |
| Eplerenone | 25 mg once | 50 mg once | |
| Inotropes | Dopamine | 5 to 10 mcg/kg/min | |
| Dobutamine | 2.5 to 5 mcg/kg/min | ||
| Milrinone | 0.125 to 0.75 mcg/kg/min | ||
| Vasodilators | Nitroglycerin | 5 to 10 mcg/min, increase dose by 5-10mcg/min every 3-5 mins as tolerated |
Max is 400mcg/min |
| Nitroprusside | 5 to 10 mcg/min, increase dose by 5-10mcg/min every 5 mins as tolerated |
Max is 400mcg/min | |
| Nesiritide | 2 mcg/kg bolus; then 0.01 mcg/kg/minute continuous infusion | Max of 0.03 mcg/kg/minute | |
| Hydralazine and isosorbide dinitrate | Fixed-dose combination | 37.5 mg hydralazine/20 mg isosorbide dinitrate 3 times daily, 75 mg hydralazine/40 mg isosorbide dinitrate 3 times daily | |
| Individual doses | Hydralazine: 25 to 50 mg 3 or 4 times daily, 300 mg daily in divided doses Isosorbide dinitrate: 20 to 30 mg 3 or 4 times daily |
120 mg daily in divided doses | |
| Digoxin | 0.125 to 0.25 mg daily. There is no need for a loading dose in CHF. Drugs that increase the concentration of digoxin include amiodarone, quinidine and verapamil |
Do's
- Guideline-directed medical therapy (GDMT) is a term which represents the optimal medical therapy in the management of heart failure as defined by ACCF/AHA. These are primarily the class 1 recommendations. It involves the use of ACE inhibitors or (ARBs), beta blockers, aldosterone antagonists, and hydralazine/nitrate medications.
- Order an echocardiogram as soon as possible in the absence of a recent one or if the patient's clinical status is deteriorating.
- Digoxin decreases hospitalization but not mortality in the RALES study. It can be used in CHF & afib to reduce the ventricular response. In the RALES study, a level of < 1 ng/ml was associated with efficacy. Levels > 1 ng/ml not associated with greater efficacy & associated with higher mortality. No need to load a CHF patient with dig. For majority of patients with normal Cr, a daily dose of 0.25 mg of digoxin is usually adequate. In the older patient or in those patients with renal impairment, a dose of 0.125 mg per day may be adequate. Drugs that increase the concentration of digoxin include amiodarone, quinidine and verapamil. [13][14][15][16][17][18][19]
- DVT prophylaxis unless contraindicated.[20][21]
- Consider adding another diuretic (e.g. metolazone or thiazides) for worsening congestion despite high doses of loop diuretics.[22][23]
- Daily serum electrolytes, urea nitrogen, and creatinine concentrations should be measured during the use of IV diuretics or active titration of heart failure medications.
- Schedule an early follow-up visit (within 7 to 14 days) and early telephone follow-up (within 3 days) of hospital discharge .[24][25]
Don'ts
- Avoid, if possible, NSAIDs, sympathomimetics, tricyclic antidepressants, class I and III antiarrhythmics (except amiodarone), and nondihydropyridine calcium channel blockers (diltiazem, verapamil as they can be harmful in acute decompensated HF. [26][27][28][29][30][31][32]
- Don't administer parenteral inotropes in normotensive patients with acute decompensated HF without evidence of decreased organ perfusion. [33]
- Don't combine an ACEI, ARB, and aldosterone antagonist in patients with HFrEF unless otherwise indicated as this combination carries a risk of renal dysfunction and hyperkalemia.
- Don't use aldosterone receptor antagonists in patients with hyperkalemia or renal insufficiency when serum creatinine is more than 2.5 mg/dL in men or more than 2.0 mg/dL in women (or estimated glomerular filtration rate <30 mL/min/1.73 m2), and/or potassium more than 5.0 mEq/L.[34][35]
- Don't use statins routinely without other indications.[36][37]
References
- ↑ 1.0 1.1 McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K; et al. (2012). "ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC". Eur Heart J. 33 (14): 1787–847. doi:10.1093/eurheartj/ehs104. PMID 22611136.
- ↑ Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH; et al. (2013). "2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines". Circulation. 128 (16): 1810–52. doi:10.1161/CIR.0b013e31829e8807. PMID 23741057.
- ↑ Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG; et al. (2009). "2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation". J Am Coll Cardiol. 53 (15): e1–e90. doi:10.1016/j.jacc.2008.11.013. PMID 19358937.
- ↑ Perna, ER.; Macín, SM.; Parras, JI.; Pantich, R.; Farías, EF.; Badaracco, JR.; Jantus, E.; Medina, F.; Brizuela, M. (2002). "Cardiac troponin T levels are associated with poor short- and long-term prognosis in patients with acute cardiogenic pulmonary edema". Am Heart J. 143 (5): 814–20. PMID 12040342. Unknown parameter
|month=ignored (help) - ↑ Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, Hetherington A; et al. (2006). "The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure". Br J Gen Pract. 56 (526): 327–33. PMC 1837840. PMID 16638247.
- ↑ Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN (2010). "HFSA 2010 Comprehensive Heart Failure Practice Guideline". Journal of Cardiac Failure. 16 (6): e1–194. doi:10.1016/j.cardfail.2010.04.004. PMID 20610207. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH; et al. (2003). "Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure". J Am Coll Cardiol. 41 (10): 1797–804. PMID 12767667.
- ↑ Metra M, Torp-Pedersen C, Cleland JG, Di Lenarda A, Komajda M, Remme WJ, Dei Cas L, Spark P, Swedberg K, Poole-Wilson PA (2007). "Should beta-blocker therapy be reduced or withdrawn after an episode of decompensated heart failure? Results from COMET". European Journal of Heart Failure. 9 (9): 901–9. doi:10.1016/j.ejheart.2007.05.011. PMID 17581778. Retrieved 2012-04-06. Unknown parameter
|month=ignored (help) - ↑ Gissi-HF Investigators. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG; et al. (2008). "Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial". Lancet. 372 (9645): 1223–30. doi:10.1016/S0140-6736(08)61239-8. PMID 18757090. Review in: Ann Intern Med. 2009 Jan 20;150(2):JC1-11
- ↑ Gheorghiade M, Gattis WA, O'Connor CM, Adams KF, Elkayam U, Barbagelata A; et al. (2004). "Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial". JAMA. 291 (16): 1963–71. doi:10.1001/jama.291.16.1963. PMID 15113814.
- ↑ Udelson JE, Smith WB, Hendrix GH, Painchaud CA, Ghazzi M, Thomas I; et al. (2001). "Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure". Circulation. 104 (20): 2417–23. PMID 11705818.
- ↑ Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM (2011). "Diuretic strategies in patients with acute decompensated heart failure". The New England Journal of Medicine. 364 (9): 797–805. doi:10.1056/NEJMoa1005419. PMC 3412356. PMID 21366472. Retrieved 2013-04-30. Unknown parameter
|month=ignored (help) - ↑ The Captopril-Digoxin Multicenter Research Group. Comparative effects of therapy with captopril and digoxin in patients with mild to moderate heart failure. JAMA. 1988;259:539–44.
- ↑ Dobbs SM, Kenyon WI, Dobbs RJ. Maintenance digoxin after an episode of heart failure: placebo-controlled trial in outpatients. Br Med J. 1977;1:749–52
- ↑ Lee DC, Johnson RA, Bingham JB, et al. Heart failure in outpatients: a randomized trial of digoxin versus placebo. N Engl J Med. 1982;306: 699–705.
- ↑ Guyatt GH, Sullivan MJ, Fallen EL, et al. A controlled trial of digoxin in congestive heart failure. Am J Cardiol. 1988;61:371–5.
- ↑ . DiBianco R, Shabetai R, Kostuk W, et al. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. N Engl J Med. 1989;320:677–83.
- ↑ Uretsky BF, Young JB, Shahidi FE, et al., for the PROVED Investigative Group. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. J Am Coll Cardiol. 1993;22:955–62.
- ↑ Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-convertingenzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1–7.
- ↑ Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A; et al. (2003). "Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study". Blood Coagul Fibrinolysis. 14 (4): 341–6. PMID 12945875.
- ↑ Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel (2012). "Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): 7S–47S. doi:10.1378/chest.1412S3. PMC 3278060. PMID 22315257.
- ↑ Grosskopf I, Rabinovitz M, Rosenfeld JB (1986). "Combination of furosemide and metolazone in the treatment of severe congestive heart failure". Isr J Med Sci. 22 (11): 787–90. PMID 3793436.
- ↑ Rosenberg J, Gustafsson F, Galatius S, Hildebrandt PR (2005). "Combination therapy with metolazone and loop diuretics in outpatients with refractory heart failure: an observational study and review of the literature". Cardiovasc Drugs Ther. 19 (4): 301–6. doi:10.1007/s10557-005-3350-2. PMID 16189620.
- ↑ Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI (2000). "Predictors of readmission among elderly survivors of admission with heart failure". Am Heart J. 139 (1 Pt 1): 72–7. PMID 10618565.
- ↑ Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW; et al. (2010). "Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure". JAMA. 303 (17): 1716–22. doi:10.1001/jama.2010.533. PMID 20442387.
- ↑ Heerdink ER, Leufkens HG, Herings RM, et al. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med. 1998;158:1108–12.
- ↑ . Herchuelz A, Derenne F, Deger F, et al. Interaction between nonsteroidal anti-inflammatory drugs and loop diuretics: modulation by sodiumbalance. J Pharmacol Exp Ther. 1989;248:1175–81.
- ↑ Gottlieb SS, Robinson S, Krichten CM, et al. Renal response to indomethacin in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70:890–3
- ↑ Bank AJ, Kubo SH, Rector TS, et al. Local forearm vasodilation with intra-arterial administration of enalaprilat in humans. Clin Pharmacol Ther. 1991;50:314–21.
- ↑ The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–12.
- ↑ The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–33.
- ↑ Pratt CM, Eaton T, Francis M, et al. The inverse relationship between baseline left ventricular ejection fraction and outcome of antiarrhythmic therapy: a dangerous imbalance in the risk-benefit ratio. Am Heart J. 1989;118:433–40.
- ↑ Cuffe MS, Califf RM, Adams KF, Benza R, Bourge R, Colucci WS, Massie BM, O'Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M (2002). "Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial". JAMA : the Journal of the American Medical Association. 287 (12): 1541–7. PMID 11911756. Retrieved 2012-04-06. Unknown parameter
|month=ignored (help) - ↑ Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A; et al. (2004). "Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study". N Engl J Med. 351 (6): 543–51. doi:10.1056/NEJMoa040135. PMID 15295047.
- ↑ Bozkurt B, Agoston I, Knowlton AA (2003). "Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines". J Am Coll Cardiol. 41 (2): 211–4. PMID 12535810.
- ↑ Horwich TB, MacLellan WR, Fonarow GC (2004). "Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure". J Am Coll Cardiol. 43 (4): 642–8. doi:10.1016/j.jacc.2003.07.049. PMID 14975476.
- ↑ Gissi-HF Investigators. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG; et al. (2008). "Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial". Lancet. 372 (9645): 1231–9. doi:10.1016/S0140-6736(08)61240-4. PMID 18757089.