Halazepam: Difference between revisions
No edit summary |
No edit summary |
||
| Line 94: | Line 94: | ||
{{Benzodiazepines}} | {{Benzodiazepines}} | ||
{{Anxiolytics}} | {{Anxiolytics}} | ||
[[Category:Benzodiazepines]] | [[Category:Benzodiazepines]] | ||
| Line 100: | Line 99: | ||
[[Category:Organofluorides]] | [[Category:Organofluorides]] | ||
[[Category:Lactams]] | [[Category:Lactams]] | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Revision as of 14:23, 13 April 2015
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | 9-chloro- 6-phenyl- 2-(2,2,2-trifluoroethyl)- 2,5-diazabicyclo[5.4.0] undeca- 5,8,10,12-tetraen -3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a684001 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | 14 hours (drug), 50-100 hours (metabolites). |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
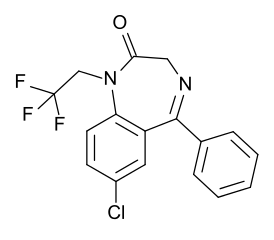
| Formula | C17H12ClF3N2O |
| Molar mass | 352.7 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Halazepam is a benzodiazepine derivative that was marketed under the brand names Paxipam in the United States,[1] Alapryl in Spain,[2] and Pacinone in Portugal.[3]
Medical uses
Halazepam was used for the treatment of anxiety.[1]
Adverse effects
Adverse effects include drowsiness, confusion, dizziness, and sedation. Gastrointestinal side effects have also been reported including dry mouth and nausea.[1]
Pharmacokinetics and pharmacodynamics
Pharmacokinetics and pharmacodynamics were listed in Current Psychotherapeutic Drugs published in June 15, 1998 as follows:[4]
| Onset of action | Intermediate to slow |
| Plasma half life | 14 hr for parent drug and 30-100 hr for its metabolite |
| Peak plasma levels | 1-3 hr for parent drug and 3-6 hf for its metabolite |
| Metabolism | Metabolized into desmethyldiazepam and 3-hydroxyhalazepam (in the liver) |
| Excretion | Excreted through kidneys |
| Protein binding | 98% bound to plasma protein |
Regulatory Information
Halazepam is classified as a schedule 4 controlled substance with a corresponding code 2762 by the Drug Enforcement Administration (DEA).[5]
Commercial production
Halazepam was invented by Schlesinger Walter in the U.S. It was marketed as an anti-anxiety agent in 1981. However, Halazepam is not commercially available in the United States because it was withdrawn by its manufacturer for poor sales.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 "halazepam". Drugs.com. Retrieved December 11, 2014.
- ↑ "Alapryl". Drugs.com. Retrieved December 11, 2014.
- ↑ "Pacinone". Drugs.com. Retrieved December 11, 2014.
- ↑ Quitkin, Frederick M. ... (1998). Current therapeutic drugs (2nd ed. ed.). Washington: American Psychiatric Press. p. 166. ISBN 0880489944.
- ↑ "SCHEDULES OF CONTROLLED SUBSTANCES". Code of Federal Reguations. 2012-04-01. pp. § 1308.14 Schedule IV. Retrieved December 12, 2014.
- Pages with script errors
- CS1 maint: Extra text
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to watched fields
- Benzodiazepines
- Organochlorides
- Organofluorides
- Lactams
- Drug