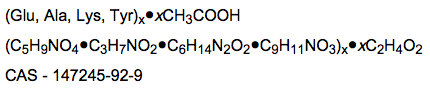Glatiramer
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Glatiramer is an immunosuppressant that is FDA approved for the {{{indicationType}}} of relapsing forms of multiple sclerosis. Common adverse reactions include injection site reactions, vasodilatation, rash, dyspnea, and chest pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Multiple Sclerosis
- Dosing Information
- Copaxone is for subcutaneous use only. Do not administer intravenously. The dosing schedule depends on the product strength that is selected. The recommended doses are:
- Copaxone 20 mg per mL: administer once per day
- Copaxone 40 mg per mL: administer three times per week and at least 48 hours apart
- Copaxone 20 mg per mL and Copaxone 40 mg per mL are not interchangeable.
- Instructions for Use
- Remove one blister-packaged prefilled syringe from the refrigerated carton. Let the prefilled syringe stand at room temperature for 20 minutes to allow the solution to warm to room temperature. Visually inspect the syringe for particulate matter and discoloration prior to administration. The solution in the syringe should appear clear, colorless to slightly yellow. If particulate matter or discoloration is observed, discard the syringe.
- Areas for subcutaneous self-injection include arms, abdomen, hips, and thighs. The prefilled syringe is for single use only. Discard unused portions.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Glatiramer in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Glatiramer in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of Copaxone have not been established in patients under 18 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Glatiramer in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Glatiramer in pediatric patients.
Contraindications
- Copaxone is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol.
Warnings
- Immediate Post-Injection Reaction
- Approximately 16% of patients exposed to Copaxone 20 mg per mL in the 5 placebo-controlled trials compared to 4% of those on placebo, and approximately 2% of patients exposed to Copaxone 40 mg per mL in a placebo-controlled trial compared to none on placebo, experienced a constellation of symptoms immediately after injection that included at least two of the following: flushing, chest pain, palpitations, anxiety, dyspnea, constriction of the throat, and urticaria. In general, these symptoms have their onset several months after the initiation of treatment, although they may occur earlier, and a given patient may experience one or several episodes of these symptoms. Whether or not any of these symptoms actually represent a specific syndrome is uncertain. Typically, the symptoms were transient and self-limited and did not require treatment; however, there have been reports of patients with similar symptoms who received emergency medical care. Whether an immunologic or nonimmunologic mechanism mediates these episodes, or whether several similar episodes seen in a given patient have identical mechanisms, is unknown.
- Chest Pain
- Approximately 13% of Copaxone 20 mg per mL patients in the 5 placebo-controlled studies compared to 6% of placebo patients, and approximately 2% of patients exposed to Copaxone 40 mg per mL in a placebo-controlled trial compared to 1% of placebo patients, experienced at least one episode of transient chest pain. While some of these episodes occurred in the context of the Immediate Post-Injection Reaction described above, many did not. The temporal relationship of this chest pain to an injection was not always known. The pain was usually transient, often unassociated with other symptoms, and appeared to have no clinical sequelae. Some patients experienced more than one such episode, and episodes usually began at least 1 month after the initiation of treatment. The pathogenesis of this symptom is unknown.
- Lipoatrophy and Skin Necrosis
- At injection sites, localized lipoatrophy and, rarely, injection site skin necrosis may occur. Lipoatrophy occurred in approximately 2% of patients exposed to Copaxone 20 mg per mL in the 5 placebo-controlled trials compared to none on placebo, and 0.5% of patients exposed to Copaxone 40 mg per mL in a single placebo-controlled trial and none on placebo. Skin necrosis has only been observed in the post-marketing setting. Lipoatrophy may occur at various times after treatment onset (sometimes after several months) and is thought to be permanent. There is no known therapy for lipoatrophy. To assist in possibly minimizing these events, the patient should be advised to follow proper injection technique and to rotate injection sites with each injection.
- Potential Effects on Immune Response
- Because Copaxone can modify immune response, it may interfere with immune functions. For example, treatment with Copaxone may interfere with the recognition of foreign antigens in a way that would undermine the body's tumor surveillance and its defenses against infection. There is no evidence that Copaxone does this, but there has not been a systematic evaluation of this risk. Because Copaxone is an antigenic material, it is possible that its use may lead to the induction of host responses that are untoward, but systematic surveillance for these effects has not been undertaken.
- Although Copaxone is intended to minimize the autoimmune response to myelin, there is the possibility that continued alteration of cellular immunity due to chronic treatment with Copaxone may result in untoward effects.
- Glatiramer acetate-reactive antibodies are formed in most patients receiving glatiramer acetate. Studies in both the rat and monkey have suggested that immune complexes are deposited in the renal glomeruli. Furthermore, in a controlled trial of 125 RRMS patients given Copaxone 20 mg per mL, subcutaneously every day for 2 years, serum IgG levels reached at least 3 times baseline values in 80% of patients by 3 months of initiation of treatment. By 12 months of treatment, however, 30% of patients still had IgG levels at least 3 times baseline values, and 90% had levels above baseline by 12 months. The antibodies are exclusively of the IgG subtype and predominantly of the IgG-1 subtype. No IgE type antibodies could be detected in any of the 94 sera tested; nevertheless, anaphylaxis can be associated with the administration of most any foreign substance, and therefore, this risk cannot be excluded.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Incidence in Controlled Clinical Trials
- COPAXONE 20 mg per mL per day
- Among 563 patients treated with COPAXONE in blinded placebo-controlled trials, approximately 5% of the subjects discontinued treatment because of an adverse reaction. The adverse reactions most commonly associated with discontinuation were: injection site reactions, dyspnea, urticaria, vasodilatation, and hypersensitivity. The most common adverse reactions were: injection site reactions, vasodilatation, rash, dyspnea, and chest pain.
- Table 1 lists treatment-emergent signs and symptoms that occurred in at least 2% of patients treated with COPAXONE 20 mg per mL in the placebo-controlled trials. These signs and symptoms were numerically more common in patients treated with COPAXONE than in patients treated with placebo. Adverse reactions were usually mild in intensity.
- Injection site atrophy comprises terms relating to localized lipoatrophy at injection site
- Adverse reactions which occurred only in 4 to 5 more subjects in the COPAXONE group than in the placebo group (less than 1% difference), but for which a relationship to COPAXONE could not be excluded, were arthralgia and herpes simplex.
- Laboratory analyses were performed on all patients participating in the clinical program for COPAXONE. Clinically-significant laboratory values for hematology, chemistry, and urinalysis were similar for both COPAXONE and placebo groups in blinded clinical trials. In controlled trials one patient discontinued treatment due to thrombocytopenia (16 x109/L), which resolved after discontinuation of treatment.
- Data on adverse reactions occurring in the controlled clinical trials of COPAXONE 20 mg per mL were analyzed to evaluate differences based on sex. No clinically-significant differences were identified. Ninety-six percent of patients in these clinical trials were Caucasian. The majority of patients treated with COPAXONE were between the ages of 18 and 45. Consequently, data are inadequate to perform an analysis of the adverse reaction incidence related to clinically-relevant age subgroups.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Glatiramer in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Glatiramer in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Glatiramer during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Glatiramer with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Glatiramer with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Glatiramer with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Glatiramer with respect to specific gender populations.
Race
There is no FDA guidance on the use of Glatiramer with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Glatiramer in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Glatiramer in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Glatiramer in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Glatiramer in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subcutaneous
Monitoring
There is limited information regarding Monitoring of Glatiramer in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Glatiramer in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Glatiramer in the drug label.
Pharmacology
Glatiramer
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | L03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 623.65 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
There is limited information regarding Glatiramer Mechanism of Action in the drug label.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Glatiramer in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Glatiramer in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Glatiramer in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Glatiramer in the drug label.
How Supplied
There is limited information regarding Glatiramer How Supplied in the drug label.
Storage
There is limited information regarding Glatiramer Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Glatiramer |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Glatiramer |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Glatiramer in the drug label.
Precautions with Alcohol
- Alcohol-Glatiramer interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Copaxone®[1]
Look-Alike Drug Names
- N/A[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Copaxone (glatiramer acetate) injection, solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Glatiramer
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Glatiramer |Label Name=Glatiramer11.png
}}
{{#subobject:
|Label Page=Glatiramer |Label Name=Glatiramer12.png
}}
{{#subobject:
|Label Page=Glatiramer |Label Name=Glatiramer13.png
}}
{{#subobject:
|Label Page=Glatiramer |Label Name=Glatiramer14.png
}}