Fosinopril: Difference between revisions
Gerald Chi- (talk | contribs) No edit summary |
Amr Marawan (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{Fosinopril}} | |||
{{drugbox | {{drugbox | ||
| IUPAC_name = 4-cyclohexyl-1-[2-[(2-methyl-1-propanoyloxy-propoxy)- (4-phenylbutyl)phosphoryl]acetyl] -pyrrolidine-2-carboxylic acid<br>(Diagrams above are fosinopril and fosinoprilat, respectively. Data below refers to fosinopril unless indicated) | | IUPAC_name = 4-cyclohexyl-1-[2-[(2-methyl-1-propanoyloxy-propoxy)- (4-phenylbutyl)phosphoryl]acetyl] -pyrrolidine-2-carboxylic acid<br>(Diagrams above are fosinopril and fosinoprilat, respectively. Data below refers to fosinopril unless indicated) | ||
| Line 22: | Line 24: | ||
| routes_of_administration = oral | | routes_of_administration = oral | ||
}} | }} | ||
{{CMG}}; {{AE}} {{AM}} | |||
'''''For patient information about Fosinopril, click [[Fosinopril (patient information)|here]].''''' | |||
{{SB}} MONOPRIL<sup>®</sup> | |||
==Overview== | ==Overview== | ||
'''Fosinopril''' is an [[ACE inhibitor|angiotensin converting enzyme (ACE) inhibitor]] used for the treatment of [[hypertension]] and some types of chronic [[heart failure]]. Fosinopril is the first and only phosphonate-containing ACE inhibitor marketed. It is marketed by [[Bristol-Myers Squibb]] under the trade name '''Monopril®'''. | '''Fosinopril''' is an [[ACE inhibitor|angiotensin converting enzyme (ACE) inhibitor]] used for the treatment of [[hypertension]] and some types of chronic [[heart failure]]. Fosinopril is the first and only phosphonate-containing ACE inhibitor marketed. It is marketed by [[Bristol-Myers Squibb]] under the trade name '''Monopril®'''. | ||
==Category== | |||
Antihypertensive Agents, ACE Inhibitors | |||
==Development== | ==Development== | ||
| Line 38: | Line 45: | ||
==Fosinoprilat and Fosinopril== | ==Fosinoprilat and Fosinopril== | ||
Fosinoprilat proved to have the same problem as enalaprilat and the other carboxylate-containing ACE inhibitors (namely poor oral [[bioavailability]]). The solution, fortunately, was very similar - the addition of a hydrophobic side-chain to modulate the ionisation characteristics of the molecule. Thus fosinopril was developed. Fosinopril is administered as a [[prodrug]] and is converted ''in vivo'' to the active form fosinoprilat. | Fosinoprilat proved to have the same problem as enalaprilat and the other carboxylate-containing ACE inhibitors (namely poor oral [[bioavailability]]). The solution, fortunately, was very similar - the addition of a hydrophobic side-chain to modulate the ionisation characteristics of the molecule. Thus fosinopril was developed. Fosinopril is administered as a [[prodrug]] and is converted ''in vivo'' to the active form fosinoprilat. | ||
==FDA Package Insert== | |||
''' [[Fosinopril indications and usage|Indications and Usage]]''' | |||
'''| [[Fosinopril dosage and administration|Dosage and Administration]]''' | |||
'''| [[Fosinopril contraindications|Contraindications]]''' | |||
'''| [[Fosinopril warnings and precautions|Warnings and Precautions]]''' | |||
'''| [[Fosinopril adverse reactions|Adverse Reactions]]''' | |||
'''| [[Fosinopril drug interactions|Drug Interactions]]''' | |||
'''| [[Fosinopril use in specific populations|Use in Specific Populations]]''' | |||
'''| [[Fosinopril overdosage|Overdosage]]''' | |||
'''| [[Fosinopril description|Description]]''' | |||
'''| [[Fosinopril clinical pharmacology|Clinical Pharmacology]]''' | |||
'''| [[Fosinopril clinical studies|Clinical Studies]]''' | |||
'''| [[Fosinopril how supplied storage and handling|How Supplied/Storage and Handling]]''' | |||
'''| [[Fosinopril patient counseling information|Patient Counseling Information]]''' | |||
'''| [[Fosinopril labels and packages|Labels and Packages]]''' | |||
{| | |||
| [[File:aaa.png|800px|thumb]] | |||
|} | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Cardiovascular Drugs]] | |||
[[Category:Drugs]] | |||
{{ACE inhibitors}} | {{ACE inhibitors}} | ||
Revision as of 15:58, 13 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2], Ahmed Zaghw, M.D. [3]
Fosinopril
Fosinopril and Hydrochlorothiazide
Overview
Captopril tablet is an angiontensin converting enzyme inhibitor drug that is FDA approved for the treatment of hypertension, heart failure, left ventricular dysfunction after myocardial infarction, diabetic nephropathy. Adverse reactions include hypotension, rash, hyperkalemia, disorder of taste, cough. hypotension, rash, hyperkalemia, disorder of taste, cough.
Category
Antihypertensive Agents, Angiotensin Converting Enzyme Inhibitors.
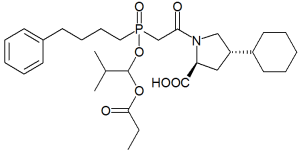 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~36% |
| Protein binding | 87% (fosinoprilat) |
| Metabolism | hepatic, GIT mucosa (to fosinoprilat) |
| Elimination half-life | 12 hours (fosinoprilat) |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C30H46NO7P |
| Molar mass | 563.663 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [5]
For patient information about Fosinopril, click here.
Synonyms / Brand Names: MONOPRIL®
Overview
Fosinopril is an angiotensin converting enzyme (ACE) inhibitor used for the treatment of hypertension and some types of chronic heart failure. Fosinopril is the first and only phosphonate-containing ACE inhibitor marketed. It is marketed by Bristol-Myers Squibb under the trade name Monopril®.
Category
Antihypertensive Agents, ACE Inhibitors
Development
The development of fosinopril started from the observation of the hypotensive effects of phosphoramidon, an extract from the bacterium Streptomyces tanashiensis. Phosphoramidon was found to be a potent inhibitor of ACE. It was speculated that the phosphoramide moiety in the molecule was central to its inhibition of ACE. Further studies found that the phosphoramide moiety served the dual-purpose of interacting with the Zn2+ in ACE, as well as mimicking the transition-state of the natural substrate of ACE.
These discoveries led to the attempt to develop a new group of ACE inhibitors which contained the phosphoramide moiety. The initial lead proved to be very potent but unstable at physiological pH. Later compounds would have a phosphonate moiety (being more stable) in place of the phosphoramide. The lessons learnt in the development of enalapril and later ACE inhibitors were applied to the design and eventually fosinoprilat was developed.
Fosinoprilat and Fosinopril
Fosinoprilat proved to have the same problem as enalaprilat and the other carboxylate-containing ACE inhibitors (namely poor oral bioavailability). The solution, fortunately, was very similar - the addition of a hydrophobic side-chain to modulate the ionisation characteristics of the molecule. Thus fosinopril was developed. Fosinopril is administered as a prodrug and is converted in vivo to the active form fosinoprilat.
FDA Package Insert
Indications and Usage | Dosage and Administration | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
References
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Pages with broken file links
- Cardiovascular Drugs
- Drugs
- ACE inhibitors