Evolocumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Evolocumab is a PCSK9 (proprotein convertase subtilisin kexin type9) inhibitor antibody that is FDA approved for the treatment of Primary Hyperlipidemia, Homozygous Familial Hypercholesterolemia, Heterozygous Familial Hypercholesterolemia, or Clinical atherosclerotic cardiovascular disease (CVD).. Common adverse reactions include Nasopharyngitis, upper respiratory tract infection, Influenza, back pain, and injection site reactions..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Evolocumab FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Evolocumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
There is limited information regarding Evolocumab Contraindications in the drug label.
Warnings
There is limited information regarding Evolocumab Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Evolocumab Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Evolocumab Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Evolocumab Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Evolocumab in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Evolocumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Evolocumab during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Evolocumab in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Evolocumab in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Evolocumab in geriatric settings.
Gender
There is no FDA guidance on the use of Evolocumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Evolocumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Evolocumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Evolocumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Evolocumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Evolocumab in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Evolocumab Administration in the drug label.
Monitoring
There is limited information regarding Evolocumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Evolocumab and IV administrations.
Overdosage
There is limited information regarding Evolocumab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Evolocumab Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Evolocumab Mechanism of Action in the drug label.
Structure
There is limited information regarding Evolocumab Structure in the drug label.
Pharmacodynamics
There is limited information regarding Evolocumab Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Evolocumab Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Evolocumab Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Evolocumab Clinical Studies in the drug label.
How Supplied
There is limited information regarding Evolocumab How Supplied in the drug label.
Storage
There is limited information regarding Evolocumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Evolocumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Evolocumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Evolocumab Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Evolocumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Evolocumab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Evolocumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
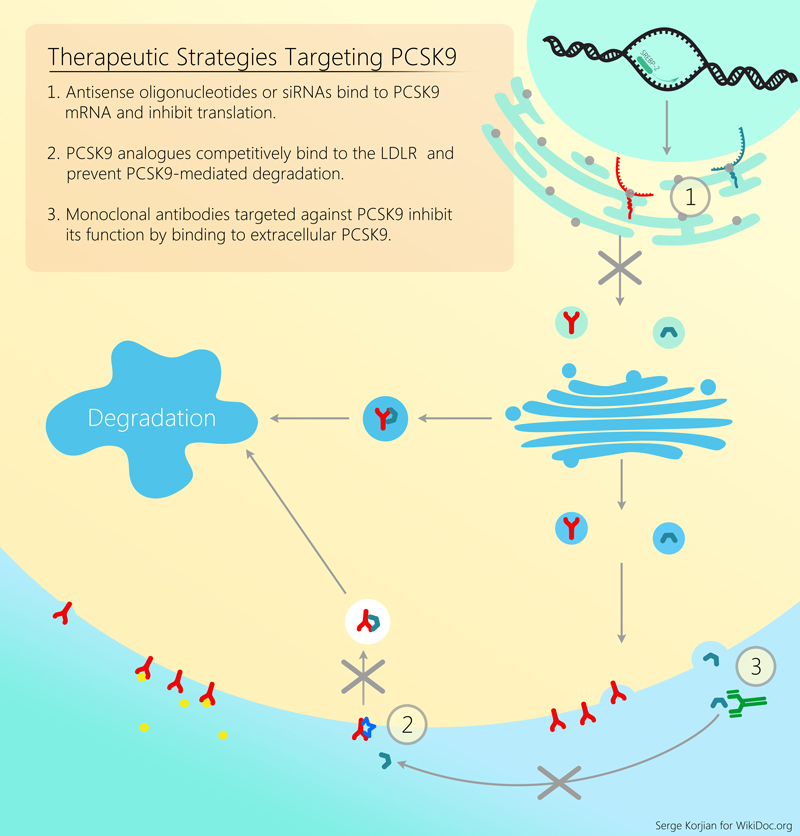
For a review of all PCSK9 inhibitors please click here
|
WikiDoc Resources for Evolocumab |
|
Articles |
|---|
|
Most recent articles on Evolocumab |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Evolocumab at Clinical Trials.gov Clinical Trials on Evolocumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Evolocumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Evolocumab Discussion groups on Evolocumab Patient Handouts on Evolocumab Directions to Hospitals Treating Evolocumab Risk calculators and risk factors for Evolocumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Evolocumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Evolocumab or AMG145 is a fully human monoclonal antibody that binds and inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) a molecule responsible for the degradation of LDL receptors. Pre-clinical studies, and early clinical trials have shown the efficacy and potency of evolocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy. Further trials are needed to evaluate the efficacy of evolocumab in improving cardiovascular outcomes.
Properties
Evolocumab aslo known as AMG145 is a fully human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is a protein that attaches to surface LDL receptors and triggers their degradation. This reduces the ability of hepatocytes to uptake LDL cholesterol and subsequently leads to increased levels of circulating LDL. By binding PCSK9, evolocumab inhibits LDL receptor destruction and increases the endocytosis of LDL from the circulation. Pre-clinical studies, and early clinical trials have shown the efficacy and potency of evolocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy.
Major Trials
Phase II Trials
RUTHERFORD
A multicenter, double-blinded, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of evolocumab in heterozygous familial hypercholesterolemia patients. 168 patients receiving statins with or without ezetimibe were randomly assigned to subcutaneous evolocumab 350 mg, 420 mg, or placebo administered every 4 weeks. At 12 weeks, LDL cholesterol was lowered by 43% and 55% with evolocumab 350 mg and 420 mg, respectively, compared with a 1% increase in the placebo group.[2]
GAUSS
Based on the fact that approximately 10% to 20% of patients cannot tolerate statins, the GAUSS trial was designed to assess the efficacy and tolerability of evolocumab in patients with statin intolerance due to muscle-related side effects. 160 patients with statin intolerance were randomized equally into 5 different groups: evolocumab alone at 280 mg, 350 mg, or 420 mg doses; evolocumab at 420 mg plus 10 mg of ezetimibe and 10 mg of ezetimibe plus placebo - all given subcutaneously. At week 12, mean LDL cholesterol levels were lowered by 41% in the lowest dose group, 51% in the highest dose group, and 63% in the high dose group of evolocumab combined with ezetimive. In comparison, 15 the placebo/ezetimibe group demonstrated a 15% decrease in LDL cholesterol. Four serious adverse events were reported with evolocumab which were coronary artery disease, acute pancreatitis, hip fracture, syncope. Myalgia was also the most common treatment-emergent adverse effect observed during the study.[3]
LAPLACE-TIMI 57
LAPLACE-TIMI 57 was designed to assess the efficacy, safety and tolerability to a range of doses of evolocumab in hypercholesterolemic patients. 631 patients on a stable dose of a statin (with or without ezetimibe) were randomly assigned to evolocumab at 70, 105, or 140 mg or placebo every two weeks or evolocumab at 280, 350, or 420 mg or placebo every four weeks. At week 12, mean LDL-C concentrations in the 2-week-dosing group was reduced from 42% to 66 % compared to a 42% to 50% reduction in the 4-week-dosing group. No serious or life-threatening events was observed.[4]
MENDEL
The MENDEL trial was designed to assess the efficacy, safety and tolerability of evolocumab as monotherapy for hypercholesterolemia. 406 untreated hypercholesterolemic patients were assigned to similar groups as in the LAPLACE-TIMI 57 study and after 12 weeks, similar results with the prior studies were obtained (39 to 51% reduction in LDL cholesterol).[5]
OSLER
OSLER was an open label study that included patients from any of the 4 phase II trials of evolocumab. It was designed to assess the efficacy and safety of longer-term (52-weeks) administration of evolocumab in patients with hypercholesterolemia. Of the initial patients enrolled in previous trials, 1104 patients (81%) elected to enroll into the OSLER study. Irrespective of initial treatment group, patients were randomized 2:1 to receive either standard of care with evolocumab 420 mg every 4 weeks or standard of care alone. Patients who received evolocumab demonstarted a 52.3% reduction in LDL choleterol levels, while patients who discontinued evolocumab and received standard of care in this trial had a return to baseline LDL levels by 12 weeks with no rebound effect. Adverse events occurred in 73.1% of patients in the standard of care arm compared to 81.4% of patients in the evolocumab arm.[6]
Phase III Trials
DESCARTES
DESCARTES compared evolocumab treatment to placebo in patients with dyslipidemia for a total duration of 52 weeks. Evolocumab or a matching placebo were administered following a run-in period of 4-12 weeks of one of four standard of care lipid-lowering therapy regimens (diet alone, diet plus 10 mg atorvastatin daily, 80 mg of atorvastatin daily, or 80 mg of atorvastatin plus 10 mg of ezetimibe daily). In total, 905 patients underwent randomization. Treatment with 420 mg of evolocumab every 4 weeks was associated with a significant reduction in LDL cholesterol levels (57% greater than placebo group). Depending on background therapy, the reductions ranged between 48.5% with 80 mg atorvastatin and ezetimibe pretreatment to 61.6% in with 10 mg atorvastatin pretreatment. Adverse events during treatment were comparable in the evolocumab group and the placebo group. The most common adverse events were nasopharyngitis, upper respiratory tract infection, influenza, and back pain. [7]
LAPLACE-2
LAPLACE-2 was designed to evaluate the efficacy and tolerability of evolocumab vs. ezetimibe when used in combination with a moderate- vs high-intensity statin. Patients (n=2067) were randomized to 1 of 24 different treatment combinations for a total duration of 12 weeks. Evolocumab was associated with a 66% to 75% reduction in LDL-C levels every 2 weeks, and a 63% to 75% reduction every monthly compared to placebo at the mean of weeks 10 and 12 in the moderate- and high-intensity statin-treated groups. Adverse events were reported in 36% of patients treated with evolocumab, compared to 40% and 39% in patients administered ezetimibe and placebo, respectively. The most common adverse events in the evolocumab treatment group were headache, back pain, muscle spasms, arthralgia, and extremity pain. [8]
MENDEL-2
MENDEL-2 randomized 614 patients to 1 of 6 combinations: oral placebo and subcutaneous (SC) placebo biweekly; oral placebo and SC placebo monthly; ezetimibe and SC placebo biweekly; ezetimibe and SC placebo monthly; oral placebo and evolocumab 140 mg biweekly; or oral placebo and evolocumab 420 mg monthly. Treatment with evolocumab led to a significant reduction in LDL-C from baseline values, on average by 55%-57% greater than placebo and by 38%-40% greater than ezetimibe. Adverse events were comparable across all treatment groups.[9]
GAUSS-2
GAUSS-2 evaluated the efficacy and safety of evolocumab compared to ezetimibe in patients with hypercholesterolemia who cannot tolerate recommended statin doses. Patients enrolled (n=307) were randomized 2:2:1:1 to evolocumab 140 mg every 2 weeks, evolocumab 420 mg once monthly, both with oral placebo, or daily oral ezetimibe with subcutaneous placebo every 2 weeks or every month. Evolocumab treatment resulted in a 53% to 56% reduction in LDL-C, corresponding to a 37% to 39% treatment difference when compared to the ezetimibe group. Adverse events led to drug discontinuation in 8% of the evolocumab-treated patients and 13% of the ezetimibe-treated patients. Myalgia occured much less often in the evolocumab treatment arms.[10]
RUTHERFORD-2
RUTHERFORD-2 was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled a total of 331 patients with heterozygous familial hypercholesterolemia unable to achieve target LDL cholesterol on statin with or without ezetimibe. Patients were randomized to one of four treatment arms: evolocumab 140 mg once every 2 weeks or placebo vs. evolocumab 420 mg once monthly or matching placebo for a total duration of 12 weeks. The coprimary endpoints were the percentage change from baseline in LDL-C at week 12 and at the mean LDL-C at week 10 and at week 12. Both doses of evolocumab achieved similar reductions in LDL-C, 61% and 56%, respectively. Evolocumab was well tolerated, with a small increase in the rate of muscle-related adverse events and nasopharyngitis.[11]
Approval and Cost-Effectiveness
Repatha (evolocumab) was approved for use in Europe in July 2015 and the FDA is scheduled to make a decision on the medication by the end of August 2015. The medication was approved for use in combination with statins or other lipid-lowering therapies in adults with FH who cannot lower their LDL sufficiently with maximum dose statins. Repatha reduced LDL levels among patients by 61% compared to standard therapy alone [12]. Analysts estimate Repatha will cost approximately $3,750 per year outside of the US and could cost upwards of $10,000 in the US. Manufacturers predict this medication will decrease medical costs by reducing the number of hospitalizations for stroke or myocardial infarction due to elevated LDL, but since the medication is intended for lifetime use, the costs are substantial. Further research into the cost-effectiveness of the drug, in terms of effectiveness in reducing atherosclerotic events and increasing quality adjusted life years, is needed.
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ Raal, F.; Scott, R.; Somaratne, R.; Bridges, I.; Li, G.; Wasserman, SM.; Stein, EA. (2012). "Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial". Circulation. 126 (20): 2408–17. doi:10.1161/CIRCULATIONAHA.112.144055. PMID 23129602. Unknown parameter
|month=ignored (help) - ↑ Sullivan, D.; Olsson, AG.; Scott, R.; Kim, JB.; Xue, A.; Gebski, V.; Wasserman, SM.; Stein, EA. (2012). "Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial". JAMA. 308 (23): 2497–506. doi:10.1001/jama.2012.25790. PMID 23128163. Unknown parameter
|month=ignored (help) - ↑ Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study". Lancet. 380 (9858): 2007–17. doi:10.1016/S0140-6736(12)61770-X. PMID 23141813.
- ↑ Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study". Lancet. 380 (9858): 1995–2006. doi:10.1016/S0140-6736(12)61771-1. PMID 23141812.
- ↑ Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G; et al. (2014). "Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial". Circulation. 129 (2): 234–43. doi:10.1161/CIRCULATIONAHA.113.007012. PMID 24255061.
- ↑ Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L; et al. (2014). "A 52-week placebo-controlled trial of evolocumab in hyperlipidemia". N Engl J Med. 370 (19): 1809–19. doi:10.1056/NEJMoa1316222. PMID 24678979.
- ↑ Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D; et al. (2014). "Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial". JAMA. 311 (18): 1870–82. doi:10.1001/jama.2014.4030. PMID 24825642.
- ↑ Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J; et al. (2014). "Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab". J Am Coll Cardiol. 63 (23): 2531–40. doi:10.1016/j.jacc.2014.03.018. PMID 24691094.
- ↑ Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF; et al. (2014). "Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab". J Am Coll Cardiol. 63 (23): 2541–8. doi:10.1016/j.jacc.2014.03.019. PMID 24694531.
- ↑ Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L; et al. (2015). "PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial". Lancet. 385 (9965): 331–40. doi:10.1016/S0140-6736(14)61399-4. PMID 25282519.
- ↑ Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J; et al. (2015). "Efficacy and safety of evolocumab in reducing lipids and cardiovascular events". N Engl J Med. 372 (16): 1500–9. doi:10.1056/NEJMoa1500858. PMID 25773607.