Evolocumab
|
WikiDoc Resources for Evolocumab |
|
Articles |
|---|
|
Most recent articles on Evolocumab |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Evolocumab at Clinical Trials.gov Clinical Trials on Evolocumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Evolocumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Evolocumab Discussion groups on Evolocumab Patient Handouts on Evolocumab Directions to Hospitals Treating Evolocumab Risk calculators and risk factors for Evolocumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Evolocumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
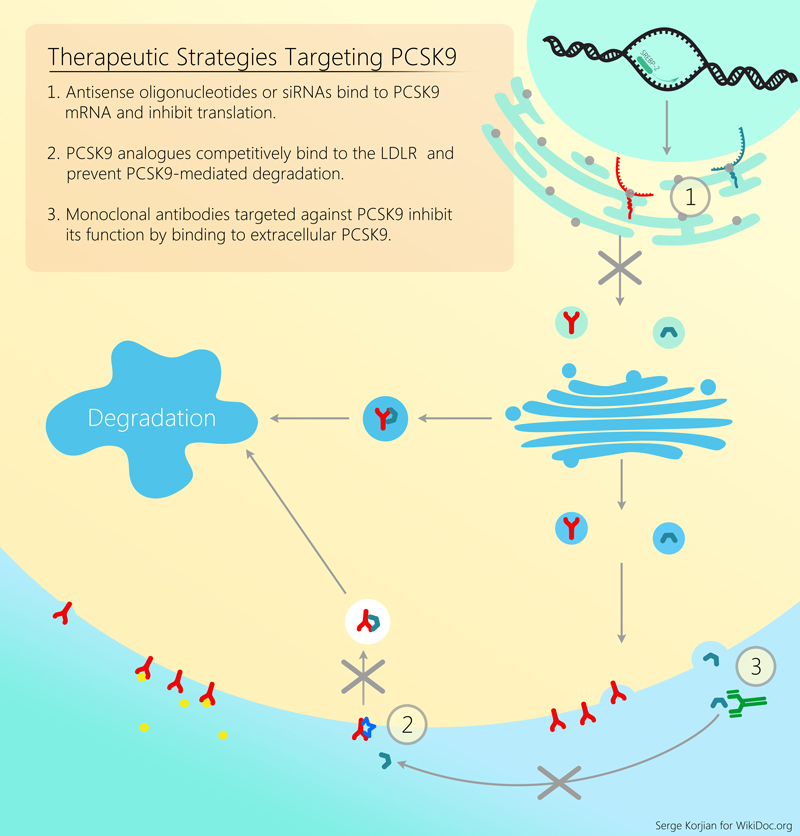
Evolocumab or AMG145 is a fully human monoclonal antibody that binds and inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) a molecule responsible for the degradation of LDL receptors. Pre-clinical studies, and early clinical trials have shown the efficacy and potency of evolocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy. Further trials are needed to evaluate the efficacy of evolocumab in improving cardiovascular outcomes.
Properties
Evolocumab aslo known as AMG145 is a fully human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is a protein that attaches to surface LDL receptors and triggers their degradation. This reduces the ability of hepatocytes to uptake LDL cholesterol and subsequently leads to increased levels of circulating LDL. By binding PCSK9, evolocumab inhibits LDL receptor destruction and increases the endocytosis of LDL from the circulation. Pre-clinical studies, and early clinical trials have shown the efficacy and potency of evolocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy.
Major Trials
Phase II Trials
RUTHERFORD
A multicenter, double-blinded, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of evolocumab in heterozygous familial hypercholesterolemia patients. 168 patients receiving statins with or without ezetimibe were randomly assigned to subcutaneous evolocumab 350 mg, AMG 145 420 mg, or placebo administered every 4 weeks. At 12 weeks, LDL cholesterol was lowered by 43% and 55% with evolocumab 350 mg and 420 mg, respectively, compared with a 1% increase in the placebo group.[2]
GAUSS
Based on the fact that approximately 10% to 20% of patients cannot tolerate statins, the GAUSS trial was designed to assess the efficacy and tolerability of evolocumab in patients with statin intolerance due to muscle-related side effects. 160 patients with statin intolerance were randomized equally into 5 different groups: evolocumab alone at 280 mg, 350 mg, or 420 mg doses; evolocumab at 420 mg plus 10 mg of ezetimibe and 10 mg of ezetimibe plus placebo - all given subcutaneously. At week 12, mean LDL cholesterol levels were lowered by 41% in the lowest dose group, 51% in the highest dose group, and 63% in the high dose group of evolocumab combined with ezetimive. In comparison, 15 the placebo/ezetimibe group demonstrated a 15% decrease in LDL cholesterol. Four serious adverse events were reported with evolocumab which were coronary artery disease, acute pancreatitis, hip fracture, syncope. Myalgia was also the most common treatment-emergent adverse effect observed during the study.[3]
LAPLACE-TIMI 57
LAPLACE-TIMI 57 was designed to assess the efficacy, safety and tolerability to a range of doses of evolocumab in hypercholesterolemic patients. 631 patients on a stable dose of a statin (with or without ezetimibe) were randomly assigned to evolocumab at 70, 105, or 140 mg or placebo every two weeks or evolocumab at 280, 350, or 420 mg or placebo every four weeks. At week 12, mean LDL-C concentrations in the 2-week-dosing group was reduced from 42% to 66 % compared to a 42% to 50% reduction in the 4-week-dosing group. No serious or life-threatening events was observed.[4]
MENDEL
The MENDEL trial was designed to assess the efficacy, safety and tolerability of evolocumab as monotherapy for hypercholesterolemia. 406 untreated hypercholesterolemic patients were assigned to similar groups as in the LAPLACE-TIMI 57 study and after 12 weeks, similar results with the prior studies were obtained (39 to 51% reduction in LDL cholesterol).[5]
OSLER
OSLER was an open label study that included patients from any of the 4 phase II trials of evolocumab. It was designed to assess the efficacy and safety of longer-term (52-weeks) administration of evolocumab in patients with hypercholesterolemia. Of the initial patients enrolled in previous trials, 1104 patients (81%) elected to enroll into the OSLER study. Irrespective of initial treatment group, patients were randomized 2:1 to receive either standard of care with evolocumab 420 mg every 4 weeks or standard of care alone. Patients who received evolocumab demonstarted a 52.3% reduction in LDL choleterol levels, while patients who discontinued evolocumab and received standard of care in this trial had a return to baseline LDL levels by 12 weeks with no rebound effect. Adverse events occurred in 73.1% of patients in the standard of care arm compared to 81.4% of patients in the evolocumab arm.[6]
Phase III Trials
LAPLACE-2
LAPLACE-2 was a phase III clinical trial designed to evaluate the efficacy and tolerability of evolocumab vs. ezetimibe when used in combination with a moderate- vs high-intensity statin. Patients (n=2067) were randomized to 1 of 24 different treatment combinations for a total duration of 12 weeks. Evolocumab was associated with a 66% to 75% reduction in LDL-C levels every 2 weeks, and a 63% to 75% reduction every monthly compared to placebo at the mean of weeks 10 and 12 in the moderate- and high-intensity statin-treated groups. Adverse events were reported in 36% of patients treated with evolocumab, compared to 40% and 39% in patients administered ezetimibe and placebo, respectively. The most common adverse events in the evolocumab treatment group were headache, back pain, muscle spasms, arthralgia, and extremity pain. [7]
MENDEL-2
GAUSS-2
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ Raal, F.; Scott, R.; Somaratne, R.; Bridges, I.; Li, G.; Wasserman, SM.; Stein, EA. (2012). "Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial". Circulation. 126 (20): 2408–17. doi:10.1161/CIRCULATIONAHA.112.144055. PMID 23129602. Unknown parameter
|month=ignored (help) - ↑ Sullivan, D.; Olsson, AG.; Scott, R.; Kim, JB.; Xue, A.; Gebski, V.; Wasserman, SM.; Stein, EA. (2012). "Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial". JAMA. 308 (23): 2497–506. doi:10.1001/jama.2012.25790. PMID 23128163. Unknown parameter
|month=ignored (help) - ↑ Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study". Lancet. 380 (9858): 2007–17. doi:10.1016/S0140-6736(12)61770-X. PMID 23141813.
- ↑ Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study". Lancet. 380 (9858): 1995–2006. doi:10.1016/S0140-6736(12)61771-1. PMID 23141812.
- ↑ Mearns BM (2014). "Dyslipidaemia: 1-Year results from OSLER trial of anti-PCSK9 monoclonal antibody evolocumab". Nat Rev Cardiol. 11 (2): 63. doi:10.1038/nrcardio.2013.201. PMID 24322554.
- ↑ Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D; et al. (2014). "Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial". JAMA. 311 (18): 1870–82. doi:10.1001/jama.2014.4030. PMID 24825642.