Evolocumab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
|indication=[[Primary Hyperlipidemia]], Homozygous [[Familial Hypercholesterolemia]], Heterozygous [[Familial Hypercholesterolemia]], or Clinical [[atherosclerotic cardiovascular disease]] (CVD). | |indication=[[Primary Hyperlipidemia]], Homozygous [[Familial Hypercholesterolemia]], Heterozygous [[Familial Hypercholesterolemia]], or Clinical [[atherosclerotic cardiovascular disease]] (CVD). | ||
|adverseReactions=[[Nasopharyngitis]], [[upper respiratory tract infection]], [[Influenza]], [[back pain]], and injection site reactions. | |adverseReactions=[[Nasopharyngitis]], [[upper respiratory tract infection]], [[Influenza]], [[back pain]], and injection site reactions. | ||
|fdaLIADAdult===== Primary Hyperlipidemia ==== | |||
* REPATHA® is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (CVD), who require additional lowering of low density lipoprotein cholesterol (LDL-C). | |||
====Homozygous Familial Hypercholesterolemia ==== | |||
* REPATHA is indicated as an adjunct to diet and other LDL-lowering therapies (e.g., statins, ezetimibe, LDL apheresis) for the treatment of patients with homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C. | |||
=== Dosage forms and strengths === | |||
* REPATHA is a sterile, clear to opalescent, colorless to pale yellow solution available as follows: | |||
** Injection: 140 mg/mL solution in a single-use profiled syringe. | |||
** Injection: 140 mg/mL solution in a single-use prefilled SureClick® auto injector. | |||
** Injection: 420 mg/3.5 mL solution in a single-use PushtronexTM system (on-body infusor with prefilled cartridge). | |||
|contraindications=* REPATHA is contraindicated in patients with a history of a serious hypersensitivity reaction to REPATHA. | |||
|warnings===== Allergic Reactions ==== | |||
* Hypersensitivity reactions (e.g., rash, urticaria) have been reported in patients treated with REPATHA, including some that led to discontinuation of therapy. | |||
* If signs or symptoms of serious allergic reactions occur, discontinue treatment with REPATHA, treat according to the standard of care, and monitor until signs and symptoms resolve. | |||
|clinicalTrials==== Clinical Trials Experience === | |||
* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
==== Adverse Reactions in Patients with Primary Hyperlipidemia and in Patients with Heterozygous Familial Hypercholesterolemia ==== | |||
* REPATHA is not indicated for use in patients without familial hypercholesterolemia or atherosclerotic CVD. | |||
* The data described below reflect exposure to REPATHA in 8 placebo-controlled trials that included 2651 patients treated with REPATHA, including 557 exposed for 6 months and 515 exposed for 1 year (median treatment duration of 12 weeks). | |||
* The mean age of the population was 57 years, 49% of the population were women, 85% White, 6% Black, 8% Asians, and 2% other races. | |||
==== Adverse Reactions in a 52-Week Controlled Trial ==== | |||
* In a 52-week, double-blind, randomized, placebo-controlled trial (Study 2), 599 patients received 420 mg of REPATHA subcutaneously once monthly. | |||
* The mean age was 56 years (range: 22 to 75 years), 23% were older than 65 years, 52% women, 80% White, 8% Black, 6% Asian, and 6% Hispanic. | |||
* Adverse reactions reported in at least 3% of REPATHA-treated patients, and more frequently than in placebo-treated patients in Study 2, are shown in Table 1. | |||
* Adverse reactions led to discontinuation of treatment in 2.2% of REPATHA-treated patients and 1% of placebo-treated patients. | |||
* The most common adverse reaction that led to REPATHA treatment discontinuation and occurred at a rate greater than placebo was myalgia (0.3% versus 0% for REPATHA and placebo, respectively). | |||
[[File:RepathaADR.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
† includes erythema, pain, bruising. | |||
==== Adverse Reactions in Seven Pooled 12-Week Controlled Trials ==== | |||
* In seven pooled 12-week, double-blind, randomized, placebo-controlled trials, 993 patients received 140 mg of REPATHA subcutaneously every 2 weeks and 1059 patients received 420 mg of REPATHA subcutaneously monthly. | |||
* The mean age was 57 years (range: 18 to 80 years), 29% were older than 65 years, 49% women, 85% White, 5% Black, 9% Asian, and 5% Hispanic. | |||
* Adverse reactions reported in at least 1% of REPATHA-treated patients, and more frequently than in placebo-treated patients, are shown in Table 2. | |||
[[File:REPATHAADR.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
==== Adverse Reactions in Eight Pooled Controlled Trials (Seven 12-Week Trials and One 52-Week Trial) ==== | |||
* The adverse reactions described below are from a pool of the 52-week trial (Study 2) and seven 12-week trials. | |||
* The mean and median exposure durations of REPATHA in this pool of eight trials were 20 weeks and 12 weeks, respectively. | |||
==== Local Injection Site Reactions ==== | |||
* Injection site reactions occurred in 3.2% and 3.0% of REPATHA-treated and placebo-treated patients, respectively. | |||
* The most common injection site reactions were erythema, pain, and bruising. | |||
* The proportions of patients who discontinued treatment due to local injection site reactions in REPATHA-treated patients and placebo-treated patients were 0.1% and 0%, respectively. | |||
==== Allergic Reactions ==== | |||
* Allergic reactions occurred in 5.1% and 4.7% of REPATHA-treated and placebo-treated patients, respectively. | |||
* The most common allergic reactions were rash (1.0% versus 0.5% for REPATHA and placebo, respectively), eczema (0.4% versus 0.2%), erythema (0.4% versus 0.2%), and urticaria (0.4% versus 0.1%). | |||
==== Neurocognitive Events ==== | |||
* In placebo-controlled trials, neurocognitive events were reported in less than or equal to 0.2% in REPATHA-treated and placebo-treated patients. | |||
==== Low LDL-C Levels ==== | |||
* In a pool of placebo- and active-controlled trials, as well as open-label extension studies that followed them, a total of 1988 patients treated with REPATHA had at least one LDL-C value < 25 mg/dL. | |||
* Changes to background lipid-altering therapy were not made in response to low LDL-C values, and REPATHA dosing was not modified or interrupted on this basis. | |||
* Although adverse consequences of very low LDL-C were not identified in these trials, the long-term effects of very low levels of LDL-C induced by REPATHA are unknown. | |||
==== Musculoskeletal Events ==== | |||
* Musculoskeletal adverse reactions were reported in 14.3% of REPATHA-treated patients and 12.8% of placebo-treated patients. | |||
* The most common adverse reactions that occurred at a rate greater than placebo were back pain (3.2% versus 2.9% for REPATHA and placebo, respectively), arthralgia (2.3% versus 2.2%), and myalgia (2.0% versus 1.8%). | |||
==== Adverse Reactions in Patients with Homozygous Familial Hypercholesterolemia ==== | |||
* In a 12-week, double-blind, randomized, placebo-controlled trial of 49 patients with HoFH (Study 4), 33 patients received 420 mg of REPATHA subcutaneously once monthly [see Clinical Studies (14.3)]. | |||
* The mean age was 31 years (range: 13 to 57 years), 49% were women, 90% White, 4% Asian, and 6% other. * The adverse reactions that occurred in at least two (6.1%) REPATHA-treated patients, and more frequently than in placebo-treated patients, included: | |||
** Upper respiratory tract infection (9.1% versus 6.3%). | |||
** Influenza (9.1% versus 0%). | |||
** Gastroenteritis (6.1% versus 0%). | |||
** Nasopharyngitis (6.1% versus 0%). | |||
=== Immunogenicity === | |||
* As with all therapeutic proteins, there is potential for immunogenicity. | |||
* The immunogenicity of REPATHA has been evaluated using an electrochemiluminescent bridging screening immunoassay for the detection of binding anti-drug antibodies. | |||
* For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies. | |||
* In a pool of placebo- and active-controlled clinical trials, 0.1% of patients treated with at least one dose of REPATHA tested positive for binding antibody development. | |||
* Patients whose sera tested positive for binding antibodies were further evaluated for neutralizing antibodies; none of the patients tested positive for neutralizing antibodies. | |||
* There was no evidence that the presence of anti-drug binding antibodies impacted the pharmacokinetic profile, clinical response, or safety of REPATHA, but the long-term consequences of continuing REPATHA treatment in the presence of anti-drug binding antibodies are unknown. | |||
* The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. | |||
* Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. | |||
* For these reasons, comparison of the incidence of antibodies to REPATHA with the incidence of antibodies to other products may be misleading. | |||
|useInPregnancyFDA==== Pregnancy === | |||
==== Risk Summary ==== | |||
* There are no data available on use of REPATHA in pregnant women to inform a drug-associated risk. | |||
* In animal reproduction studies, there were no effects on pregnancy or neonatal/infant development when monkeys were subcutaneously administered evolocumab from organogenesis through parturition at dose exposures up to 12 times the exposure at the maximum recommended human dose of 420 mg every month. | |||
* In a similar study with another drug in the PCSK9 inhibitor antibody class, humoral immune suppression was observed in infant monkeys exposed to that drug in utero at all doses. | |||
* The exposures where immune suppression occurred in infant monkeys were greater than those expected clinically. | |||
* No assessment for immune suppression was conducted with evolocumab in infant monkeys. | |||
* Measurable evolocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that evolocumab, like other IgG antibodies, crosses the placental barrier. | |||
* FDA’s experience with monoclonal antibodies in humans indicates that they are unlikely to cross the placenta in the first trimester; however, they are likely to cross the placenta in increasing amounts in the second and third trimester. | |||
* Consider the benefits and risks of REPATHA and possible risks to the fetus before prescribing REPATHA to pregnant women. | |||
* In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. | |||
==== Data ==== | |||
===== Animal Data ===== | |||
* In cynomolgus monkeys, no effects on embryo-fetal or postnatal development (up to 6 months of age) were observed when evolocumab was dosed during organogenesis to parturition at 50 mg/kg once every 2 weeks by the subcutaneous route at exposures 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
* No test of humoral immunity in infant monkeys was conducted with evolocumab. | |||
|useInNursing==== Lactation === | |||
==== Risk Summary ==== | |||
* There is no information regarding the presence of evolocumab in human milk, the effects on the breastfed infant, or the effects on milk production. | |||
* The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for REPATHA and any potential adverse effects on the breastfed infant from REPATHA or from the underlying maternal condition. | |||
* Human IgG is present in human milk, but published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. | |||
|useInPed==== Pediatric Use === | |||
* The safety and effectiveness of REPATHA in combination with diet and other LDL-C-lowering therapies in adolescents with HoFH who require additional lowering of LDL-C were established based on data from a 12-week, placebo-controlled trial that included 10 adolescents (ages 13 to 17 years old) with HoFH. | |||
* In this trial, 7 adolescents received REPATHA 420 mg subcutaneously once monthly and 3 adolescents received placebo. | |||
* The effect of REPATHA on LDL-C was generally similar to that observed among adult patients with HoFH. | |||
* Including experience from open-label, uncontrolled studies, a total of 14 adolescents with HoFH have been treated with REPATHA, with a median exposure duration of 9 months. | |||
* The safety profile of REPATHA in these adolescents was similar to that described for adult patients with HoFH. | |||
* The safety and effectiveness of REPATHA have not been established in pediatric patients with HoFH who are younger than 13 years old. | |||
* The safety and effectiveness of REPATHA have not been established in pediatric patients with primary hyperlipidemia or HeFH. | |||
|useInGeri==== Geriatric Use === | |||
* In controlled studies, 1420 patients treated with REPATHA were ≥ 65 years old and 171 were ≥ 75 years old. | |||
* No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |||
|useInRenalImpair==== Renal Impairment === | |||
* No dose adjustment is needed in patients with mild to moderate renal impairment. No data are available in patients with severe renal impairment. | |||
|useInHepaticImpair==== Hepatic Impairment === | |||
* No dose adjustment is needed in patients with mild to moderate hepatic impairment (Child-Pugh A or B). | |||
* No data are available in patients with severe hepatic impairment. | |||
}} | }} | ||
__NOTOC__ | __NOTOC__ | ||
Revision as of 14:11, 2 March 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Evolocumab is a PCSK9 (proprotein convertase subtilisin kexin type9) inhibitor antibody that is FDA approved for the treatment of Primary Hyperlipidemia, Homozygous Familial Hypercholesterolemia, Heterozygous Familial Hypercholesterolemia, or Clinical atherosclerotic cardiovascular disease (CVD).. Common adverse reactions include Nasopharyngitis, upper respiratory tract infection, Influenza, back pain, and injection site reactions..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Primary Hyperlipidemia
- REPATHA® is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (CVD), who require additional lowering of low density lipoprotein cholesterol (LDL-C).
Homozygous Familial Hypercholesterolemia
- REPATHA is indicated as an adjunct to diet and other LDL-lowering therapies (e.g., statins, ezetimibe, LDL apheresis) for the treatment of patients with homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C.
Dosage forms and strengths
- REPATHA is a sterile, clear to opalescent, colorless to pale yellow solution available as follows:
- Injection: 140 mg/mL solution in a single-use profiled syringe.
- Injection: 140 mg/mL solution in a single-use prefilled SureClick® auto injector.
- Injection: 420 mg/3.5 mL solution in a single-use PushtronexTM system (on-body infusor with prefilled cartridge).
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Evolocumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
- REPATHA is contraindicated in patients with a history of a serious hypersensitivity reaction to REPATHA.
Warnings
Allergic Reactions
- Hypersensitivity reactions (e.g., rash, urticaria) have been reported in patients treated with REPATHA, including some that led to discontinuation of therapy.
- If signs or symptoms of serious allergic reactions occur, discontinue treatment with REPATHA, treat according to the standard of care, and monitor until signs and symptoms resolve.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Patients with Primary Hyperlipidemia and in Patients with Heterozygous Familial Hypercholesterolemia
- REPATHA is not indicated for use in patients without familial hypercholesterolemia or atherosclerotic CVD.
- The data described below reflect exposure to REPATHA in 8 placebo-controlled trials that included 2651 patients treated with REPATHA, including 557 exposed for 6 months and 515 exposed for 1 year (median treatment duration of 12 weeks).
- The mean age of the population was 57 years, 49% of the population were women, 85% White, 6% Black, 8% Asians, and 2% other races.
Adverse Reactions in a 52-Week Controlled Trial
- In a 52-week, double-blind, randomized, placebo-controlled trial (Study 2), 599 patients received 420 mg of REPATHA subcutaneously once monthly.
- The mean age was 56 years (range: 22 to 75 years), 23% were older than 65 years, 52% women, 80% White, 8% Black, 6% Asian, and 6% Hispanic.
- Adverse reactions reported in at least 3% of REPATHA-treated patients, and more frequently than in placebo-treated patients in Study 2, are shown in Table 1.
- Adverse reactions led to discontinuation of treatment in 2.2% of REPATHA-treated patients and 1% of placebo-treated patients.
- The most common adverse reaction that led to REPATHA treatment discontinuation and occurred at a rate greater than placebo was myalgia (0.3% versus 0% for REPATHA and placebo, respectively).

† includes erythema, pain, bruising.
Adverse Reactions in Seven Pooled 12-Week Controlled Trials
- In seven pooled 12-week, double-blind, randomized, placebo-controlled trials, 993 patients received 140 mg of REPATHA subcutaneously every 2 weeks and 1059 patients received 420 mg of REPATHA subcutaneously monthly.
- The mean age was 57 years (range: 18 to 80 years), 29% were older than 65 years, 49% women, 85% White, 5% Black, 9% Asian, and 5% Hispanic.
- Adverse reactions reported in at least 1% of REPATHA-treated patients, and more frequently than in placebo-treated patients, are shown in Table 2.

Adverse Reactions in Eight Pooled Controlled Trials (Seven 12-Week Trials and One 52-Week Trial)
- The adverse reactions described below are from a pool of the 52-week trial (Study 2) and seven 12-week trials.
- The mean and median exposure durations of REPATHA in this pool of eight trials were 20 weeks and 12 weeks, respectively.
Local Injection Site Reactions
- Injection site reactions occurred in 3.2% and 3.0% of REPATHA-treated and placebo-treated patients, respectively.
- The most common injection site reactions were erythema, pain, and bruising.
- The proportions of patients who discontinued treatment due to local injection site reactions in REPATHA-treated patients and placebo-treated patients were 0.1% and 0%, respectively.
Allergic Reactions
- Allergic reactions occurred in 5.1% and 4.7% of REPATHA-treated and placebo-treated patients, respectively.
- The most common allergic reactions were rash (1.0% versus 0.5% for REPATHA and placebo, respectively), eczema (0.4% versus 0.2%), erythema (0.4% versus 0.2%), and urticaria (0.4% versus 0.1%).
Neurocognitive Events
- In placebo-controlled trials, neurocognitive events were reported in less than or equal to 0.2% in REPATHA-treated and placebo-treated patients.
Low LDL-C Levels
- In a pool of placebo- and active-controlled trials, as well as open-label extension studies that followed them, a total of 1988 patients treated with REPATHA had at least one LDL-C value < 25 mg/dL.
- Changes to background lipid-altering therapy were not made in response to low LDL-C values, and REPATHA dosing was not modified or interrupted on this basis.
- Although adverse consequences of very low LDL-C were not identified in these trials, the long-term effects of very low levels of LDL-C induced by REPATHA are unknown.
Musculoskeletal Events
- Musculoskeletal adverse reactions were reported in 14.3% of REPATHA-treated patients and 12.8% of placebo-treated patients.
- The most common adverse reactions that occurred at a rate greater than placebo were back pain (3.2% versus 2.9% for REPATHA and placebo, respectively), arthralgia (2.3% versus 2.2%), and myalgia (2.0% versus 1.8%).
Adverse Reactions in Patients with Homozygous Familial Hypercholesterolemia
- In a 12-week, double-blind, randomized, placebo-controlled trial of 49 patients with HoFH (Study 4), 33 patients received 420 mg of REPATHA subcutaneously once monthly [see Clinical Studies (14.3)].
- The mean age was 31 years (range: 13 to 57 years), 49% were women, 90% White, 4% Asian, and 6% other. * The adverse reactions that occurred in at least two (6.1%) REPATHA-treated patients, and more frequently than in placebo-treated patients, included:
- Upper respiratory tract infection (9.1% versus 6.3%).
- Influenza (9.1% versus 0%).
- Gastroenteritis (6.1% versus 0%).
- Nasopharyngitis (6.1% versus 0%).
Immunogenicity
- As with all therapeutic proteins, there is potential for immunogenicity.
- The immunogenicity of REPATHA has been evaluated using an electrochemiluminescent bridging screening immunoassay for the detection of binding anti-drug antibodies.
- For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
- In a pool of placebo- and active-controlled clinical trials, 0.1% of patients treated with at least one dose of REPATHA tested positive for binding antibody development.
- Patients whose sera tested positive for binding antibodies were further evaluated for neutralizing antibodies; none of the patients tested positive for neutralizing antibodies.
- There was no evidence that the presence of anti-drug binding antibodies impacted the pharmacokinetic profile, clinical response, or safety of REPATHA, but the long-term consequences of continuing REPATHA treatment in the presence of anti-drug binding antibodies are unknown.
- The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay.
- Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
- For these reasons, comparison of the incidence of antibodies to REPATHA with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Evolocumab Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Evolocumab Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy
Risk Summary
- There are no data available on use of REPATHA in pregnant women to inform a drug-associated risk.
- In animal reproduction studies, there were no effects on pregnancy or neonatal/infant development when monkeys were subcutaneously administered evolocumab from organogenesis through parturition at dose exposures up to 12 times the exposure at the maximum recommended human dose of 420 mg every month.
- In a similar study with another drug in the PCSK9 inhibitor antibody class, humoral immune suppression was observed in infant monkeys exposed to that drug in utero at all doses.
- The exposures where immune suppression occurred in infant monkeys were greater than those expected clinically.
- No assessment for immune suppression was conducted with evolocumab in infant monkeys.
- Measurable evolocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that evolocumab, like other IgG antibodies, crosses the placental barrier.
- FDA’s experience with monoclonal antibodies in humans indicates that they are unlikely to cross the placenta in the first trimester; however, they are likely to cross the placenta in increasing amounts in the second and third trimester.
- Consider the benefits and risks of REPATHA and possible risks to the fetus before prescribing REPATHA to pregnant women.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
- In cynomolgus monkeys, no effects on embryo-fetal or postnatal development (up to 6 months of age) were observed when evolocumab was dosed during organogenesis to parturition at 50 mg/kg once every 2 weeks by the subcutaneous route at exposures 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
- No test of humoral immunity in infant monkeys was conducted with evolocumab.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Evolocumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Evolocumab during labor and delivery.
Nursing Mothers
Lactation
Risk Summary
- There is no information regarding the presence of evolocumab in human milk, the effects on the breastfed infant, or the effects on milk production.
- The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for REPATHA and any potential adverse effects on the breastfed infant from REPATHA or from the underlying maternal condition.
- Human IgG is present in human milk, but published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts.
Pediatric Use
Pediatric Use
- The safety and effectiveness of REPATHA in combination with diet and other LDL-C-lowering therapies in adolescents with HoFH who require additional lowering of LDL-C were established based on data from a 12-week, placebo-controlled trial that included 10 adolescents (ages 13 to 17 years old) with HoFH.
- In this trial, 7 adolescents received REPATHA 420 mg subcutaneously once monthly and 3 adolescents received placebo.
- The effect of REPATHA on LDL-C was generally similar to that observed among adult patients with HoFH.
- Including experience from open-label, uncontrolled studies, a total of 14 adolescents with HoFH have been treated with REPATHA, with a median exposure duration of 9 months.
- The safety profile of REPATHA in these adolescents was similar to that described for adult patients with HoFH.
- The safety and effectiveness of REPATHA have not been established in pediatric patients with HoFH who are younger than 13 years old.
- The safety and effectiveness of REPATHA have not been established in pediatric patients with primary hyperlipidemia or HeFH.
Geriatic Use
Geriatric Use
- In controlled studies, 1420 patients treated with REPATHA were ≥ 65 years old and 171 were ≥ 75 years old.
- No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Evolocumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Evolocumab with respect to specific racial populations.
Renal Impairment
Renal Impairment
- No dose adjustment is needed in patients with mild to moderate renal impairment. No data are available in patients with severe renal impairment.
Hepatic Impairment
Hepatic Impairment
- No dose adjustment is needed in patients with mild to moderate hepatic impairment (Child-Pugh A or B).
- No data are available in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Evolocumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Evolocumab in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Evolocumab Administration in the drug label.
Monitoring
There is limited information regarding Evolocumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Evolocumab and IV administrations.
Overdosage
There is limited information regarding Evolocumab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Evolocumab Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Evolocumab Mechanism of Action in the drug label.
Structure
There is limited information regarding Evolocumab Structure in the drug label.
Pharmacodynamics
There is limited information regarding Evolocumab Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Evolocumab Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Evolocumab Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Evolocumab Clinical Studies in the drug label.
How Supplied
There is limited information regarding Evolocumab How Supplied in the drug label.
Storage
There is limited information regarding Evolocumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Evolocumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Evolocumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Evolocumab Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Evolocumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Evolocumab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Evolocumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
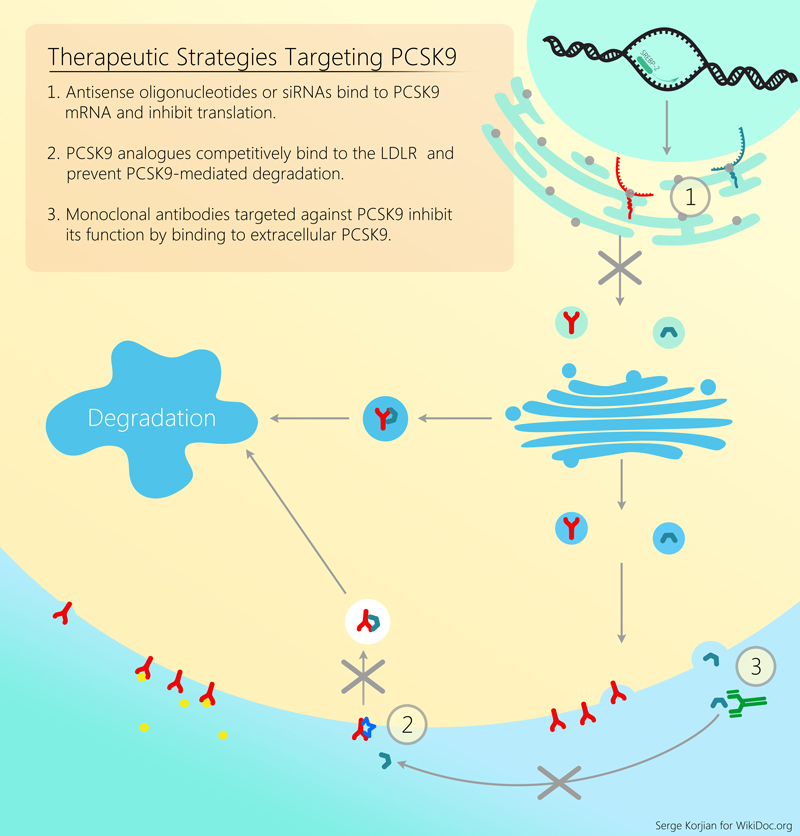
For a review of all PCSK9 inhibitors please click here
|
WikiDoc Resources for Evolocumab |
|
Articles |
|---|
|
Most recent articles on Evolocumab |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Evolocumab at Clinical Trials.gov Clinical Trials on Evolocumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Evolocumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Evolocumab Discussion groups on Evolocumab Patient Handouts on Evolocumab Directions to Hospitals Treating Evolocumab Risk calculators and risk factors for Evolocumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Evolocumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Evolocumab or AMG145 is a fully human monoclonal antibody that binds and inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) a molecule responsible for the degradation of LDL receptors. Pre-clinical studies, and early clinical trials have shown the efficacy and potency of evolocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy. Further trials are needed to evaluate the efficacy of evolocumab in improving cardiovascular outcomes.
Properties
Evolocumab aslo known as AMG145 is a fully human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is a protein that attaches to surface LDL receptors and triggers their degradation. This reduces the ability of hepatocytes to uptake LDL cholesterol and subsequently leads to increased levels of circulating LDL. By binding PCSK9, evolocumab inhibits LDL receptor destruction and increases the endocytosis of LDL from the circulation. Pre-clinical studies, and early clinical trials have shown the efficacy and potency of evolocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy.
Major Trials
Phase II Trials
RUTHERFORD
A multicenter, double-blinded, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of evolocumab in heterozygous familial hypercholesterolemia patients. 168 patients receiving statins with or without ezetimibe were randomly assigned to subcutaneous evolocumab 350 mg, 420 mg, or placebo administered every 4 weeks. At 12 weeks, LDL cholesterol was lowered by 43% and 55% with evolocumab 350 mg and 420 mg, respectively, compared with a 1% increase in the placebo group.[2]
GAUSS
Based on the fact that approximately 10% to 20% of patients cannot tolerate statins, the GAUSS trial was designed to assess the efficacy and tolerability of evolocumab in patients with statin intolerance due to muscle-related side effects. 160 patients with statin intolerance were randomized equally into 5 different groups: evolocumab alone at 280 mg, 350 mg, or 420 mg doses; evolocumab at 420 mg plus 10 mg of ezetimibe and 10 mg of ezetimibe plus placebo - all given subcutaneously. At week 12, mean LDL cholesterol levels were lowered by 41% in the lowest dose group, 51% in the highest dose group, and 63% in the high dose group of evolocumab combined with ezetimive. In comparison, 15 the placebo/ezetimibe group demonstrated a 15% decrease in LDL cholesterol. Four serious adverse events were reported with evolocumab which were coronary artery disease, acute pancreatitis, hip fracture, syncope. Myalgia was also the most common treatment-emergent adverse effect observed during the study.[3]
LAPLACE-TIMI 57
LAPLACE-TIMI 57 was designed to assess the efficacy, safety and tolerability to a range of doses of evolocumab in hypercholesterolemic patients. 631 patients on a stable dose of a statin (with or without ezetimibe) were randomly assigned to evolocumab at 70, 105, or 140 mg or placebo every two weeks or evolocumab at 280, 350, or 420 mg or placebo every four weeks. At week 12, mean LDL-C concentrations in the 2-week-dosing group was reduced from 42% to 66 % compared to a 42% to 50% reduction in the 4-week-dosing group. No serious or life-threatening events was observed.[4]
MENDEL
The MENDEL trial was designed to assess the efficacy, safety and tolerability of evolocumab as monotherapy for hypercholesterolemia. 406 untreated hypercholesterolemic patients were assigned to similar groups as in the LAPLACE-TIMI 57 study and after 12 weeks, similar results with the prior studies were obtained (39 to 51% reduction in LDL cholesterol).[5]
OSLER
OSLER was an open label study that included patients from any of the 4 phase II trials of evolocumab. It was designed to assess the efficacy and safety of longer-term (52-weeks) administration of evolocumab in patients with hypercholesterolemia. Of the initial patients enrolled in previous trials, 1104 patients (81%) elected to enroll into the OSLER study. Irrespective of initial treatment group, patients were randomized 2:1 to receive either standard of care with evolocumab 420 mg every 4 weeks or standard of care alone. Patients who received evolocumab demonstarted a 52.3% reduction in LDL choleterol levels, while patients who discontinued evolocumab and received standard of care in this trial had a return to baseline LDL levels by 12 weeks with no rebound effect. Adverse events occurred in 73.1% of patients in the standard of care arm compared to 81.4% of patients in the evolocumab arm.[6]
Phase III Trials
DESCARTES
DESCARTES compared evolocumab treatment to placebo in patients with dyslipidemia for a total duration of 52 weeks. Evolocumab or a matching placebo were administered following a run-in period of 4-12 weeks of one of four standard of care lipid-lowering therapy regimens (diet alone, diet plus 10 mg atorvastatin daily, 80 mg of atorvastatin daily, or 80 mg of atorvastatin plus 10 mg of ezetimibe daily). In total, 905 patients underwent randomization. Treatment with 420 mg of evolocumab every 4 weeks was associated with a significant reduction in LDL cholesterol levels (57% greater than placebo group). Depending on background therapy, the reductions ranged between 48.5% with 80 mg atorvastatin and ezetimibe pretreatment to 61.6% in with 10 mg atorvastatin pretreatment. Adverse events during treatment were comparable in the evolocumab group and the placebo group. The most common adverse events were nasopharyngitis, upper respiratory tract infection, influenza, and back pain. [7]
LAPLACE-2
LAPLACE-2 was designed to evaluate the efficacy and tolerability of evolocumab vs. ezetimibe when used in combination with a moderate- vs high-intensity statin. Patients (n=2067) were randomized to 1 of 24 different treatment combinations for a total duration of 12 weeks. Evolocumab was associated with a 66% to 75% reduction in LDL-C levels every 2 weeks, and a 63% to 75% reduction every monthly compared to placebo at the mean of weeks 10 and 12 in the moderate- and high-intensity statin-treated groups. Adverse events were reported in 36% of patients treated with evolocumab, compared to 40% and 39% in patients administered ezetimibe and placebo, respectively. The most common adverse events in the evolocumab treatment group were headache, back pain, muscle spasms, arthralgia, and extremity pain. [8]
MENDEL-2
MENDEL-2 randomized 614 patients to 1 of 6 combinations: oral placebo and subcutaneous (SC) placebo biweekly; oral placebo and SC placebo monthly; ezetimibe and SC placebo biweekly; ezetimibe and SC placebo monthly; oral placebo and evolocumab 140 mg biweekly; or oral placebo and evolocumab 420 mg monthly. Treatment with evolocumab led to a significant reduction in LDL-C from baseline values, on average by 55%-57% greater than placebo and by 38%-40% greater than ezetimibe. Adverse events were comparable across all treatment groups.[9]
GAUSS-2
GAUSS-2 evaluated the efficacy and safety of evolocumab compared to ezetimibe in patients with hypercholesterolemia who cannot tolerate recommended statin doses. Patients enrolled (n=307) were randomized 2:2:1:1 to evolocumab 140 mg every 2 weeks, evolocumab 420 mg once monthly, both with oral placebo, or daily oral ezetimibe with subcutaneous placebo every 2 weeks or every month. Evolocumab treatment resulted in a 53% to 56% reduction in LDL-C, corresponding to a 37% to 39% treatment difference when compared to the ezetimibe group. Adverse events led to drug discontinuation in 8% of the evolocumab-treated patients and 13% of the ezetimibe-treated patients. Myalgia occured much less often in the evolocumab treatment arms.[10]
RUTHERFORD-2
RUTHERFORD-2 was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled a total of 331 patients with heterozygous familial hypercholesterolemia unable to achieve target LDL cholesterol on statin with or without ezetimibe. Patients were randomized to one of four treatment arms: evolocumab 140 mg once every 2 weeks or placebo vs. evolocumab 420 mg once monthly or matching placebo for a total duration of 12 weeks. The coprimary endpoints were the percentage change from baseline in LDL-C at week 12 and at the mean LDL-C at week 10 and at week 12. Both doses of evolocumab achieved similar reductions in LDL-C, 61% and 56%, respectively. Evolocumab was well tolerated, with a small increase in the rate of muscle-related adverse events and nasopharyngitis.[11]
Approval and Cost-Effectiveness
Repatha (evolocumab) was approved for use in Europe in July 2015 and the FDA is scheduled to make a decision on the medication by the end of August 2015. The medication was approved for use in combination with statins or other lipid-lowering therapies in adults with FH who cannot lower their LDL sufficiently with maximum dose statins. Repatha reduced LDL levels among patients by 61% compared to standard therapy alone [12]. Analysts estimate Repatha will cost approximately $3,750 per year outside of the US and could cost upwards of $10,000 in the US. Manufacturers predict this medication will decrease medical costs by reducing the number of hospitalizations for stroke or myocardial infarction due to elevated LDL, but since the medication is intended for lifetime use, the costs are substantial. Further research into the cost-effectiveness of the drug, in terms of effectiveness in reducing atherosclerotic events and increasing quality adjusted life years, is needed.
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ Raal, F.; Scott, R.; Somaratne, R.; Bridges, I.; Li, G.; Wasserman, SM.; Stein, EA. (2012). "Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial". Circulation. 126 (20): 2408–17. doi:10.1161/CIRCULATIONAHA.112.144055. PMID 23129602. Unknown parameter
|month=ignored (help) - ↑ Sullivan, D.; Olsson, AG.; Scott, R.; Kim, JB.; Xue, A.; Gebski, V.; Wasserman, SM.; Stein, EA. (2012). "Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial". JAMA. 308 (23): 2497–506. doi:10.1001/jama.2012.25790. PMID 23128163. Unknown parameter
|month=ignored (help) - ↑ Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study". Lancet. 380 (9858): 2007–17. doi:10.1016/S0140-6736(12)61770-X. PMID 23141813.
- ↑ Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study". Lancet. 380 (9858): 1995–2006. doi:10.1016/S0140-6736(12)61771-1. PMID 23141812.
- ↑ Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G; et al. (2014). "Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial". Circulation. 129 (2): 234–43. doi:10.1161/CIRCULATIONAHA.113.007012. PMID 24255061.
- ↑ Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L; et al. (2014). "A 52-week placebo-controlled trial of evolocumab in hyperlipidemia". N Engl J Med. 370 (19): 1809–19. doi:10.1056/NEJMoa1316222. PMID 24678979.
- ↑ Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D; et al. (2014). "Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial". JAMA. 311 (18): 1870–82. doi:10.1001/jama.2014.4030. PMID 24825642.
- ↑ Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J; et al. (2014). "Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab". J Am Coll Cardiol. 63 (23): 2531–40. doi:10.1016/j.jacc.2014.03.018. PMID 24691094.
- ↑ Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF; et al. (2014). "Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab". J Am Coll Cardiol. 63 (23): 2541–8. doi:10.1016/j.jacc.2014.03.019. PMID 24694531.
- ↑ Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L; et al. (2015). "PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial". Lancet. 385 (9965): 331–40. doi:10.1016/S0140-6736(14)61399-4. PMID 25282519.
- ↑ Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J; et al. (2015). "Efficacy and safety of evolocumab in reducing lipids and cardiovascular events". N Engl J Med. 372 (16): 1500–9. doi:10.1056/NEJMoa1500858. PMID 25773607.