Everolimus: Difference between revisions
No edit summary |
No edit summary |
||
| Line 90: | Line 90: | ||
:*Table 1 summarizes recommendations for dose reduction, interruption or discontinuation of AFINITOR in the management of adverse reactions. General management recommendations are also provided as applicable. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment. | :*Table 1 summarizes recommendations for dose reduction, interruption or discontinuation of AFINITOR in the management of adverse reactions. General management recommendations are also provided as applicable. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment. | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Hepatic Impairment | *Hepatic Impairment | ||
| Line 338: | Line 326: | ||
*Table 2 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥10% for patients receiving AFINITOR 10 mg daily versus placebo. | *Table 2 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥10% for patients receiving AFINITOR 10 mg daily versus placebo. | ||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Key observed laboratory abnormalities are presented in Table 3. | *Key observed laboratory abnormalities are presented in Table 3. | ||
: [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Clinical Study Experience in Advanced Pancreatic Neuroendocrine Tumors===== | =====Clinical Study Experience in Advanced Pancreatic Neuroendocrine Tumors===== | ||
| Line 354: | Line 340: | ||
*Table 4 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo. | *Table 4 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo. | ||
: [[File:{{PAGENAME}}04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*In female patients aged 18 to 55 years, irregular menstruation occurred in 5 of 46 (11%) AFINITOR-treated females and none of the 33 females in the placebo group. | *In female patients aged 18 to 55 years, irregular menstruation occurred in 5 of 46 (11%) AFINITOR-treated females and none of the 33 females in the placebo group. | ||
| Line 361: | Line 346: | ||
*Key observed laboratory abnormalities are presented in Table 5. | *Key observed laboratory abnormalities are presented in Table 5. | ||
: [[File:{{PAGENAME}}05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Clinical Study Experience in Advanced Renal Cell Carcinoma===== | =====Clinical Study Experience in Advanced Renal Cell Carcinoma===== | ||
| Line 372: | Line 356: | ||
*Table 6 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo. Within each MedDRA system organ class, the adverse reactions are presented in order of decreasing frequency. | *Table 6 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo. Within each MedDRA system organ class, the adverse reactions are presented in order of decreasing frequency. | ||
: [[File:{{PAGENAME}}06.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Other notable adverse reactions occurring more frequently with AFINITOR than with placebo, but with an incidence of < 10% include: | *Other notable adverse reactions occurring more frequently with AFINITOR than with placebo, but with an incidence of < 10% include: | ||
| Line 392: | Line 375: | ||
*Key laboratory abnormalities are presented in Table 7. | *Key laboratory abnormalities are presented in Table 7. | ||
: [[File:{{PAGENAME}}07.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Clinical Study Experience in Renal Angiomyolipoma with Tuberous Sclerosis Complex===== | =====Clinical Study Experience in Renal Angiomyolipoma with Tuberous Sclerosis Complex===== | ||
| Line 405: | Line 387: | ||
*Table 8 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 9. | *Table 8 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 9. | ||
: [[File:{{PAGENAME}}08.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Amenorrhea occurred in 15% of AFINITOR-treated females (8 of 52) and 4% (1 of 26) of females in the placebo group. Other adverse reactions involving the female reproductive system were menorrhagia (10%), menstrual irregularities (10%), and vaginal hemorrhage (8%). | *Amenorrhea occurred in 15% of AFINITOR-treated females (8 of 52) and 4% (1 of 26) of females in the placebo group. Other adverse reactions involving the female reproductive system were menorrhagia (10%), menstrual irregularities (10%), and vaginal hemorrhage (8%). | ||
| Line 415: | Line 393: | ||
*The following additional adverse reactions occurred in less than 10% of Afinitor-treated patients: epistaxis (9%), decreased appetite (6%), otitis media (6%), depression (5%), abnormal taste (5%), increased blood luteinizing hormone (LH) levels (4%), increased blood follicle stimulating hormone (FSH) levels (3%), hypersensitivity (3%), and pneumonitis (1%). | *The following additional adverse reactions occurred in less than 10% of Afinitor-treated patients: epistaxis (9%), decreased appetite (6%), otitis media (6%), depression (5%), abnormal taste (5%), increased blood luteinizing hormone (LH) levels (4%), increased blood follicle stimulating hormone (FSH) levels (3%), hypersensitivity (3%), and pneumonitis (1%). | ||
: [[File:{{PAGENAME}}09.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Clinical Study Experience in Subependymal Giant Cell Astrocytoma with Tuberous Sclerosis Complex===== | =====Clinical Study Experience in Subependymal Giant Cell Astrocytoma with Tuberous Sclerosis Complex===== | ||
| Line 430: | Line 405: | ||
*Table 10 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 11. | *Table 10 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 11. | ||
: [[File:{{PAGENAME}}10.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Amenorrhea occurred in 17% of AFINITOR-treated females aged 10 to 55 years (3 of 18) and none of the females in the placebo group. For this same group of AFINITOR-treated females, the following menstrual abnormalities were reported: dysmenorrhea (6%), menorrhagia (6%), metrorrhagia (6%), and unspecified menstrual irregularity (6%). | *Amenorrhea occurred in 17% of AFINITOR-treated females aged 10 to 55 years (3 of 18) and none of the females in the placebo group. For this same group of AFINITOR-treated females, the following menstrual abnormalities were reported: dysmenorrhea (6%), menorrhagia (6%), metrorrhagia (6%), and unspecified menstrual irregularity (6%). | ||
| Line 437: | Line 411: | ||
*The following additional adverse reactions occurred in less than 10% of AFINITOR-treated patients: nausea (8%), pain in extremity (8%), insomnia (6%), pneumonia (6%), epistaxis (5%), hypersensitivity (3%), increased blood luteinizing hormone (LH) levels (1%) and pneumonitis (1%). | *The following additional adverse reactions occurred in less than 10% of AFINITOR-treated patients: nausea (8%), pain in extremity (8%), insomnia (6%), pneumonia (6%), epistaxis (5%), hypersensitivity (3%), increased blood luteinizing hormone (LH) levels (1%) and pneumonitis (1%). | ||
: [[File:{{PAGENAME}}11.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Longer-term follow-up of 34.2 months (range 4.7 to 47.1 months) from a non-randomized, open-label, 28-patient trial resulted in the following additional notable adverse reactions and key laboratory abnormalities: cellulitis (29%), hyperglycemia (25%), and elevated creatinine (14%). | *Longer-term follow-up of 34.2 months (range 4.7 to 47.1 months) from a non-randomized, open-label, 28-patient trial resulted in the following additional notable adverse reactions and key laboratory abnormalities: cellulitis (29%), hyperglycemia (25%), and elevated creatinine (14%). | ||
| Line 661: | Line 635: | ||
*The molecular formula is C53H83NO14 and the molecular weight is 958.2. The structural formula is: | *The molecular formula is C53H83NO14 and the molecular weight is 958.2. The structural formula is: | ||
: [[File:{{PAGENAME}} | : [[File:{{PAGENAME}}28.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*AFINITOR Tablets are supplied for oral administration and contain 2.5 mg, 5 mg, 7.5 mg, or 10 mg of everolimus. The tablets also contain anhydrous lactose, butylated hydroxytoluene, crospovidone, hypromellose, lactose monohydrate, and magnesium stearate as inactive ingredients. | *AFINITOR Tablets are supplied for oral administration and contain 2.5 mg, 5 mg, 7.5 mg, or 10 mg of everolimus. The tablets also contain anhydrous lactose, butylated hydroxytoluene, crospovidone, hypromellose, lactose monohydrate, and magnesium stearate as inactive ingredients. | ||
| Line 753: | Line 727: | ||
: [[File:{{PAGENAME}}12.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:{{PAGENAME}}29.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Advanced Neuroendocrine Tumors===== | =====Advanced Neuroendocrine Tumors===== | ||
| Line 766: | Line 741: | ||
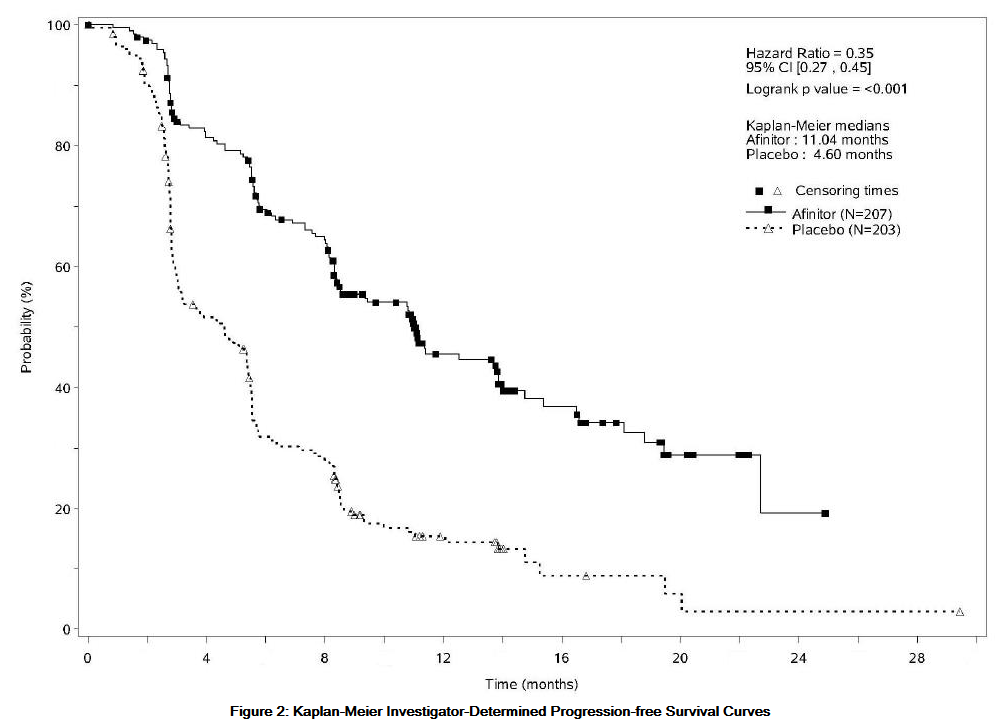
*The trial demonstrated a statistically significant improvement in PFS (median 11.0 months versus 4.6 months), resulting in a 65% risk reduction in investigator-determined PFS (HR 0.35; 95%CI: 0.27 to 0.45; p<0.001) (see Table 13 and Figure 2). PFS improvement was observed across all patient subgroups, irrespective of prior somatostatin analog use. The PFS results by investigator radiological review, central radiological review and adjudicated radiological review are shown below in Table 13. | *The trial demonstrated a statistically significant improvement in PFS (median 11.0 months versus 4.6 months), resulting in a 65% risk reduction in investigator-determined PFS (HR 0.35; 95%CI: 0.27 to 0.45; p<0.001) (see Table 13 and Figure 2). PFS improvement was observed across all patient subgroups, irrespective of prior somatostatin analog use. The PFS results by investigator radiological review, central radiological review and adjudicated radiological review are shown below in Table 13. | ||
: [[File:{{PAGENAME}}13.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:{{PAGENAME}}30.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Investigator-determined response rate was low (4.8%) in the AFINITOR arm and there were no complete responses. The overall survival results are not yet mature and no statistically significant treatment-related difference in OS was noted [HR=1.05 (95% CI: 0.71 to 1.55)]. | *Investigator-determined response rate was low (4.8%) in the AFINITOR arm and there were no complete responses. The overall survival results are not yet mature and no statistically significant treatment-related difference in OS was noted [HR=1.05 (95% CI: 0.71 to 1.55)]. | ||
| Line 787: | Line 761: | ||
*AFINITOR was superior to placebo for PFS (see Table 14 and Figure 3). The treatment effect was similar across prognostic scores and prior sorafenib and/or sunitinib. Final overall survival (OS) results yield a hazard ratio of 0.90 (95% CI: 0.71 to 1.14), with no statistically significant difference between the 2 treatment groups. Planned cross-over from placebo due to disease progression to open label AFINITOR occurred in 111 of the 139 patients (79.9%) and may have confounded the OS benefit. | *AFINITOR was superior to placebo for PFS (see Table 14 and Figure 3). The treatment effect was similar across prognostic scores and prior sorafenib and/or sunitinib. Final overall survival (OS) results yield a hazard ratio of 0.90 (95% CI: 0.71 to 1.14), with no statistically significant difference between the 2 treatment groups. Planned cross-over from placebo due to disease progression to open label AFINITOR occurred in 111 of the 139 patients (79.9%) and may have confounded the OS benefit. | ||
: [[File:{{PAGENAME}}14.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:{{PAGENAME}}31.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Renal Angiomyolipoma with Tuberous Sclerosis Complex===== | =====Renal Angiomyolipoma with Tuberous Sclerosis Complex===== | ||
| Line 802: | Line 775: | ||
*The renal angiomyolipoma response rate was statistically significantly higher in AFINITOR-treated patients; there were 33 (41.8%) patients with angiomyolipoma responses in the AFINITOR arm as compared to none in the placebo arm. Results are displayed in Table 15. The median response duration was 5.3+ months (range 2.3+ to 19.6+ months). | *The renal angiomyolipoma response rate was statistically significantly higher in AFINITOR-treated patients; there were 33 (41.8%) patients with angiomyolipoma responses in the AFINITOR arm as compared to none in the placebo arm. Results are displayed in Table 15. The median response duration was 5.3+ months (range 2.3+ to 19.6+ months). | ||
: [[File:{{PAGENAME}}15.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*There were 3 patients in the AFINITOR arm and 8 patients in the placebo arm with documented angiomyolipoma progression by central radiologic review. The time to angiomyolipoma progression was statistically significantly longer in the AFINITOR arm (HR 0.08 [95% CI: 0.02, 0.37]; p <0.0001). | *There were 3 patients in the AFINITOR arm and 8 patients in the placebo arm with documented angiomyolipoma progression by central radiologic review. The time to angiomyolipoma progression was statistically significantly longer in the AFINITOR arm (HR 0.08 [95% CI: 0.02, 0.37]; p <0.0001). | ||
| Line 818: | Line 791: | ||
*The SEGA response rate was statistically significantly higher in AFINITOR-treated patients. There were 27 (35%) patients with SEGA responses in the AFINITOR arm and no SEGA responses in the placebo arm. Results are displayed in Table 16. At the time of the final analysis, all SEGA responses were ongoing and the median duration of response was 5.3 months (range 2.1 to 8.4 months). No patient in either treatment arm required surgical intervention during the course of Study 1. | *The SEGA response rate was statistically significantly higher in AFINITOR-treated patients. There were 27 (35%) patients with SEGA responses in the AFINITOR arm and no SEGA responses in the placebo arm. Results are displayed in Table 16. At the time of the final analysis, all SEGA responses were ongoing and the median duration of response was 5.3 months (range 2.1 to 8.4 months). No patient in either treatment arm required surgical intervention during the course of Study 1. | ||
: [[File:{{PAGENAME}}16.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*With a median follow-up of 8.4 months, SEGA progression was detected in 6 of 39 (15.4%) patients randomized to receive placebo and none of the 78 patients randomized to receive AFINITOR. | *With a median follow-up of 8.4 months, SEGA progression was detected in 6 of 39 (15.4%) patients randomized to receive placebo and none of the 78 patients randomized to receive AFINITOR. | ||
| Line 918: | Line 892: | ||
: [[File:{{PAGENAME}}17.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:{{PAGENAME}}18.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
| Line 967: | Line 939: | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}19.png|This image is provided by the National Library of Medicine. | ||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}20.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}21.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}22.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}23.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}24.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}25.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}26.png|This image is provided by the National Library of Medicine. | |||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}27.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
| Line 977: | Line 977: | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:]] | |||
Revision as of 16:23, 20 August 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Everolimus is a kinase inhibitor that is FDA approved for the {{{indicationType}}} of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane after failure of treatment with letrozole or anastrozole, adults with progressive neuroendocrine tumors of pancreatic origin (PNET) that are unresectable, locally advanced or metastatic. AFINITOR is not indicated for the treatment of patients with functional carcinoid tumors, adults with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib, adults with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery.. Common adverse reactions include stomatitis, infections, rash, fatigue, diarrhea, edema, abdominal pain, nausea, fever, asthenia, cough, headache and decreased appetite.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer (Advanced HR+ BC)
- AFINITOR® is indicated for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane, after failure of treatment with letrozole or anastrozole.
Advanced Neuroendocrine Tumors of Pancreatic Origin (PNET)
- AFINITOR® is indicated for the treatment of adult patients with progressive neuroendocrine tumors of pancreatic origin (PNET) with unresectable, locally advanced or metastatic disease.
- AFINITOR® is not indicated for the treatment of patients with functional carcinoid tumors.
Advanced Renal Cell Carcinoma (RCC)
- AFINITOR® is indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib.
Renal Angiomyolipoma with Tuberous Sclerosis Complex (TSC)
- AFINITOR® is indicated for the treatment of adult patients with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery.
- The effectiveness of AFINITOR in the treatment of renal angiomyolipoma is based on an analysis of durable objective responses in patients treated for a median of 8.3 months. Further follow-up of patients is required to determine long-term outcomes.
Subependymal Giant Cell Astrocytoma (SEGA) with Tuberous Sclerosis Complex (TSC)
- AFINITOR® Tablets and AFINITOR® DISPERZ are indicated in pediatric and adult patients with tuberous sclerosis complex (TSC) for the treatment of subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected.
- The effectiveness of AFINITOR Tablets and AFINITOR DISPERZ is based on demonstration of durable objective response, as evidenced by reduction in SEGA tumor volume. Improvement in disease-related symptoms and overall survival in patients with SEGA and TSC has not been demonstrated [see Clinical Studies (14.5)].
DOSAGE AND ADMINISTRATION
- AFINITOR is available in two dosage forms: tablets (AFINITOR Tablets) and tablets for oral suspension (AFINITOR DISPERZ).
- AFINITOR Tablets may be used for all approved indications.
- AFINITOR DISPERZ is approved for the treatment of patients with subependymal giant cell astrocytoma (SEGA) and tuberous sclerosis complex (TSC).
Recommended Dose in Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer, Advanced PNET, Advanced RCC, and Renal Angiomyolipoma with TSC
- The recommended dose of AFINITOR Tablets is 10 mg, to be taken once daily at the same time every day. Administer either consistently with food or consistently without food [see Clinical Pharmacology (12.3)]. AFINITOR Tablets should be swallowed whole with a glass of water. Do not break or crush tablets.
- Continue treatment until disease progression or unacceptable toxicity occurs.
Dose Modifications in Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer, Advanced PNET, Advanced RCC, and Renal Angiomyolipoma with TSC
- Adverse Reactions
- Management of severe or intolerable adverse reactions may require temporary dose interruption (with or without a dose reduction of AFINITOR therapy) or discontinuation. If dose reduction is required, the suggested dose is approximately 50% lower than the daily dose previously administered [see Warnings and Precautions (5)].
- Table 1 summarizes recommendations for dose reduction, interruption or discontinuation of AFINITOR in the management of adverse reactions. General management recommendations are also provided as applicable. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment.
- Hepatic Impairment
- Hepatic impairment will increase the exposure to everolimus [see Warnings and Precautions (5.9) and Use in Specific Populations (8.8)]. Dose adjustments are recommended:
- Mild hepatic impairment (Child-Pugh class A) – The recommended dose is 7.5 mg daily; the dose may be decreased to 5 mg if not well tolerated.
- Moderate hepatic impairment (Child-Pugh class B) – The recommended dose is 5 mg daily; the dose may be decreased to 2.5 mg if not well tolerated.
- Severe hepatic impairment (Child-Pugh class C) – If the desired benefit outweighs the risk, a dose of 2.5 mg daily may be used but must not be exceeded.
- Dose adjustments should be made if a patient’s hepatic (Child-Pugh) status changes during treatment.
- CYP3A4/P-glycoprotein (PgP) Inhibitors
- Avoid the use of strong CYP3A4/PgP inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole) [see Warnings and Precautions (5.8) and Drug Interactions (7.1)].
- Use caution when co-administered with moderate CYP3A4/PgP inhibitors (e.g., amprenavir, fosamprenavir, aprepitant, erythromycin, fluconazole, verapamil, diltiazem). If patients require co-administration of a moderate CYP3A4/PgP inhibitor, reduce the AFINITOR dose to 2.5 mg daily. The reduced dose of AFINITOR is predicted to adjust the area under the curve (AUC) to the range observed without inhibitors. An AFINITOR dose increase from 2.5 mg to 5 mg may be considered based on patient tolerance. If the moderate inhibitor is discontinued, a washout period of approximately 2 to 3 days should be allowed before the AFINITOR dose is increased. If the moderate inhibitor is discontinued, the AFINITOR dose should be returned to the dose used prior to initiation of the moderate CYP3A4/PgP inhibitor.
- Grapefruit, grapefruit juice, and other foods that are known to inhibit cytochrome P450 and PgP activity may increase everolimus exposures and should be avoided during treatment.
- Strong CYP3A4/PgP Inducers
- Avoid the use of concomitant strong CYP3A4/PgP inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital). If patients require co-administration of a strong CYP3A4/PgP inducer, consider doubling the daily dose of AFINITOR using increments of 5 mg or less. This dose of AFINITOR is predicted, based on pharmacokinetic data, to adjust the AUC to the range observed without inducers. However, there are no clinical data with this dose adjustment in patients receiving strong CYP3A4/PgP inducers. If the strong inducer is discontinued, consider a washout period of 3 to 5 days, before the AFINITOR dose is returned to the dose used prior to initiation of the strong CYP3A4/PgP inducer [see Warnings and Precautions (5.8) and Drug Interactions (7.2)].
- St. John’s Wort (Hypericum perforatum) may decrease everolimus exposure unpredictably and should be avoided.
Recommended Dose in SEGA with TSC
- The recommended starting dose is 4.5 mg/m2, once daily. The recommended starting dose for patients with severe hepatic impairment (Child-Pugh class C) or requiring moderate CYP3A4/PgP inhibitors is 2.5 mg/m2, once daily [see Dosage and Administration (2.5)]. The recommended starting dose for patients requiring a concomitant strong CYP3A4 inducer is 9 mg/m2, once daily [see Dosage and Administration (2.5)]. Round dose to the nearest strength of either AFINITOR Tablets or AFINITOR DISPERZ.
- Do not combine AFINITOR Tablets and AFINITOR DISPERZ to achieve the desired total dose.
- Use therapeutic drug monitoring to guide subsequent dosing [see Dosage and Administration (2.4)]. Adjust dose at 2 week intervals as needed to achieve and maintain trough concentrations of 5 to 15 ng/mL [see Dosage and Administration (2.4, 2.5)].
- Continue treatment until disease progression or unacceptable toxicity occurs. The optimal duration of therapy is unknown.
Therapeutic Drug Monitoring in SEGA with TSC
- Monitor everolimus whole blood trough levels routinely in all patients. When possible, use the same assay and laboratory for therapeutic drug monitoring throughout treatment.
- Assess trough concentrations approximately 2 weeks after initiation of treatment, a change in dose, a change in co-administration of CYP3A4/PgP inducers and/or inhibitors, a change in hepatic function, or a change in dosage form between AFINITOR Tablets and AFINITOR DISPERZ. Once a stable dose is attained, monitor trough concentrations every 3 to 6 months in patients with changing body surface area or every 6 to 12 months in patients with stable body surface area for the duration of treatment.
- Titrate the dose to attain trough concentrations of 5 to 15 ng/mL.
- For trough concentrations less than 5 ng/mL, increase the daily dose by 2.5 mg (in patients taking AFINITOR Tablets) or 2 mg (in patients taking AFINITOR DISPERZ).
- For trough concentrations greater than 15 ng/mL, reduce the daily dose by 2.5 mg (in patients taking AFINITOR Tablets) or 2 mg (in patients taking AFINITOR DISPERZ).
- If dose reduction is required for patients receiving the lowest available strength, administer every other day.
Dose Modifications in SEGA with TSC
- Adverse Reactions
- Temporarily interrupt or permanently discontinue AFINITOR Tablets or AFINITOR DISPERZ for severe or intolerable adverse reactions. If dose reduction is required when reinitiating therapy, reduce the dose by approximately 50% [see Dosage and Administration (2.2) and Warnings and Precautions (5)]. If dose reduction is required for patients receiving the lowest available strength, administer every other day.
- Hepatic Impairment
- Reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50% in patients with SEGA who have severe hepatic impairment (Child-Pugh class C) [see Dosage and Administration (2.3)]. Adjustment to the starting dose for patients with SEGA who have mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment may not be needed. Subsequent dosing should be based on therapeutic drug monitoring.
- Assess everolimus trough concentrations approximately 2 weeks after commencing treatment, a change in dose, or any change in hepatic function [see Dosage and Administration (2.3, 2.4)].
- CYP3A4/P-glycoprotein (PgP) Inhibitors
- Avoid the use of concomitant strong CYP3A4/PgP inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole) in patients receiving AFINITOR Tablets or AFINITOR DISPERZ [see Warnings and Precautions (5.8) and Drug Interactions (7.1)].
- For patients who require treatment with moderate CYP3A4/PgP inhibitors (e.g., amprenavir, fosamprenavir, aprepitant, erythromycin, fluconazole, verapamil, diltiazem):
- Reduce the AFINITOR Tablets or AFINITOR DISPERZ dose by approximately 50%. Administer every other day if dose reduction is required for patients receiving the lowest available strength and maintain trough concentrations of 5 to 15 ng/mL [see Dosage and Administration (2.3, 2.4)].
- Assess everolimus trough concentrations approximately 2 weeks after dose reduction [see Dosage and Administration (2.3, 2.4)].
- Resume the dose that was used prior to initiating the CYP3A4/PgP inhibitor 2 to 3 days after discontinuation of a moderate inhibitor. Assess the everolimus trough concentration approximately 2 weeks later [see Dosage and Administration (2.3, 2.4)].
- Do not ingest foods or nutritional supplements (e.g., grapefruit, grapefruit juice) that are known to inhibit cytochrome P450 or PgP activity.
- Strong CYP3A4/PgP Inducers
- Avoid the use of concomitant strong CYP3A4/PgP inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital) if alternative therapy is available [see Warnings and Precautions (5.8) and Drug Interactions (7.2)]. For patients who require treatment with a strong CYP3A4/PgP inducer:
- Double the dose of AFINITOR Tablets or AFINITOR DISPERZ and assess tolerability [see Dosage and Administration (2.3)].
- Assess the everolimus trough concentration 2 weeks after doubling the dose and adjust the dose if necessary to maintain a trough concentration of 5 to 15 ng/mL [see Dosage and Administration (2.3, 2.4)].
- Return the AFINITOR Tablets or AFINITOR DISPERZ dose to that used prior to initiating the strong CYP3A4/PgP inducer if the strong inducer is discontinued, and assess the everolimus trough concentrations approximately 2 weeks later [see Dosage and Administration (2.3, 2.4)].
- Do not ingest foods or nutritional supplements (e.g., St. John’s Wort (Hypericum perforatum)) that are known to induce cytochrome P450 activity.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Everolimus in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Everolimus in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Everolimus in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Everolimus in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Everolimus in pediatric patients.
Contraindications
- AFINITOR is contraindicated in patients with hypersensitivity to the active substance, to other rapamycin derivatives, or to any of the excipients. Hypersensitivity reactions manifested by symptoms including, but not limited to, anaphylaxis, dyspnea, flushing, chest pain, or angioedema (e.g., swelling of the airways or tongue, with or without respiratory impairment) have been observed with everolimus and other rapamycin derivatives.
Warnings
Precautions
- Non-infectious Pneumonitis
- Non-infectious pneumonitis is a class effect of rapamycin derivatives, including AFINITOR. Non-infectious pneumonitis was reported in up to 19% of patients treated with AFINITOR in clinical trials. The incidence of Common Terminology Criteria (CTC) Grade 3 and 4 non-infectious pneumonitis was up to 4.0% and up to 0.2%, respectively [see Adverse Reactions (6.1, 6.2, 6.3, 6.4, 6.5)]. Fatal outcomes have been observed.
- Consider a diagnosis of non-infectious pneumonitis in patients presenting with non-specific respiratory signs and symptoms such as hypoxia, pleural effusion, cough, or dyspnea, and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations. Opportunistic infections such as pneumocystis jiroveci pneumonia (PJP) should be considered in the differential diagnosis. Advise patients to report promptly any new or worsening respiratory symptoms.
- Patients who develop radiological changes suggestive of non-infectious pneumonitis and have few or no symptoms may continue AFINITOR therapy without dose alteration. Imaging appears to overestimate the incidence of clinical pneumonitis.
- If symptoms are moderate, consider interrupting therapy until symptoms improve. The use of corticosteroids may be indicated. AFINITOR may be reintroduced at a daily dose approximately 50% lower than the dose previously administered [see Table 1 in Dosage and Administration (2.2)].
- For cases of Grade 3 non-infectious pneumonitis interrupt AFINITOR until resolution to less than or equal to Grade 1. AFINITOR may be re-introduced at a daily dose approximately 50% lower than the dose previously administered depending on the individual clinical circumstances [see Dosage and Administration (2.2)]. If toxicity recurs at Grade 3, consider discontinuation of AFINITOR. For cases of Grade 4 non-infectious pneumonitis, discontinue AFINITOR. Corticosteroids may be indicated until clinical symptoms resolve. For patients who require use of corticosteroids for treatment of non-infectious pneumonitis, prophylaxis for PJP may be considered. The development of pneumonitis has been reported even at a reduced dose.
- Infections
- AFINITOR has immunosuppressive properties and may predispose patients to bacterial, fungal, viral, or protozoal infections, including infections with opportunistic pathogens [see Adverse Reactions (6.1, 6.2, 6.3, 6.4, 6.5)]. Localized and systemic infections, including pneumonia, mycobacterial infections, other bacterial infections, invasive fungal infections, such as aspergillosis, candidiasis, or pneumocystis jiroveci pneumonia (PJP) and viral infections including reactivation of hepatitis B virus have occurred in patients taking AFINITOR. Some of these infections have been severe (e.g., leading to sepsis, respiratory or hepatic failure) or fatal. Physicians and patients should be aware of the increased risk of infection with AFINITOR. Complete treatment of pre-existing invasive fungal infections prior to starting treatment with AFINITOR. While taking AFINITOR, be vigilant for signs and symptoms of infection; if a diagnosis of an infection is made, institute appropriate treatment promptly and consider interruption or discontinuation of AFINITOR. If a diagnosis of invasive systemic fungal infection is made, discontinue AFINITOR and treat with appropriate antifungal therapy.
- Pneumocystis jiroveci pneumonia, some with a fatal outcome, has been reported in patients who received everolimus. This may be associated with concomitant use of corticosteroids or other immunosuppressive agents. Prophylaxis for PJP should be considered when concomitant use of corticosteroids or other immunosuppressive agents are required.
- Oral Ulceration
- Mouth ulcers, stomatitis, and oral mucositis have occurred in patients treated with AFINITOR at an incidence ranging from 44%-78% across the clinical trial experience. Grade 3 or 4 stomatitis was reported in 4%-9% of patients [see Adverse Reactions (6.1, 6.2, 6.3, 6.4, 6.5)]. In such cases, topical treatments are recommended, but alcohol-, hydrogen peroxide-, iodine-, or thyme- containing mouthwashes should be avoided as they may exacerbate the condition. Antifungal agents should not be used unless fungal infection has been diagnosed [see Drug Interactions (7.1)].
- Renal Failure
- Cases of renal failure (including acute renal failure), some with a fatal outcome, have been observed in patients treated with AFINITOR [see Laboratory Tests and Monitoring (5.7)].
- Impaired Wound Healing
- Everolimus delays wound healing and increases the occurrence of wound-related complications like wound dehiscence, wound infection, incisional hernia, lymphocele, and seroma. These wound-related complications may require surgical intervention. Exercise caution with the use of AFINITOR in the peri-surgical period.
- Geriatric Patients
- In the randomized advanced hormone receptor-positive, HER2-negative breast cancer study, the incidence of deaths due to any cause within 28 days of the last AFINITOR dose was 6% in patients ≥ 65 years of age compared to 2% in patients < 65 years of age. Adverse reactions leading to permanent treatment discontinuation occurred in 33% of patients ≥ 65 years of age compared to 17% in patients < 65 years of age. Careful monitoring and appropriate dose adjustments for adverse reactions are recommended [see Dosage and Administration (2.2), Use in Specific Populations (8.5)].
- Laboratory Tests and Monitoring
- Renal Function
- Elevations of serum creatinine and proteinuria have been reported in patients taking AFINITOR [see Adverse Reactions (6.1, 6.2, 6.3, 6.4, 6.5)]. Monitoring of renal function, including measurement of blood urea nitrogen (BUN), urinary protein, or serum creatinine, is recommended prior to the start of AFINITOR therapy and periodically thereafter. Renal function of patients should be monitored particularly where patients have additional risk factors that may further impair renal function.
- Blood Glucose and Lipids
- Hyperglycemia, hyperlipidemia, and hypertriglyceridemia have been reported in patients taking AFINITOR [see Adverse Reactions (6.1, 6.2, 6.3, 6.4, 6.5)]. Monitoring of fasting serum glucose and lipid profile is recommended prior to the start of AFINITOR therapy and periodically thereafter as well as management with appropriate medical therapy. More frequent monitoring is recommended when AFINITOR is co-administered with other drugs that may induce hyperglycemia. When possible, optimal glucose and lipid control should be achieved before starting a patient on AFINITOR.
- Hematologic Parameters
- Decreased hemoglobin, lymphocytes, neutrophils, and platelets have been reported in patients taking AFINITOR [see Adverse Reactions (6.1, 6.2, 6.3, 6.4, 6.5)]. Monitoring of complete blood count is recommended prior to the start of AFINITOR therapy and periodically thereafter.
- Drug-drug Interactions
- Due to significant increases in exposure of everolimus, co-administration with strong CYP3A4/PgP inhibitors should be avoided [see Dosage and Administration (2.2, 2.5) and Drug Interactions (7.1)].
- A reduction of the AFINITOR dose is recommended when co-administered with a moderate CYP3A4/PgP inhibitor [see Dosage and Administration (2.2, 2.5) and Drug Interactions (7.1)].
- An increase in the AFINITOR dose is recommended when co-administered with a strong CYP3A4/PgP inducer [see Dosage and Administration (2.2, 2.5) and Drug Interactions (7.2)].
- Hepatic Impairment
- Exposure to everolimus was increased in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
- For advanced HR+ BC, advanced PNET, advanced RCC, and renal angiomyolipoma with TSC patients with severe hepatic impairment (Child-Pugh class C), AFINITOR may be used at a reduced dose if the desired benefit outweighs the risk. For patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, a dose reduction is recommended [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
- For patients with SEGA and mild or moderate hepatic impairment, adjust the dose of AFINITOR Tablets or AFINITOR DISPERZ based on therapeutic drug monitoring. For patients with SEGA and severe hepatic impairment, reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50% and adjust subsequent doses based on therapeutic drug monitoring [see Dosage and Administration (2.4, 2.5)].
- Vaccinations
- During AFINITOR treatment, avoid the use of live vaccines and avoid close contact with individuals who have received live vaccines (e.g., intranasal influenza, measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid vaccines).
- For pediatric patients with SEGA that do not require immediate treatment, complete the recommended childhood series of live virus vaccinations according to American Council on Immunization Practices (ACIP) guidelines prior to the start of therapy. An accelerated vaccination schedule may be appropriate.
- Embryo-fetal Toxicity
- Based on the mechanism of action, AFINITOR can cause fetal harm. Everolimus caused embryo-fetal toxicities in animals at maternal exposures that were lower than human exposures. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Use in Specific Populations (8.1)].
- Advise female patients of reproductive potential to avoid becoming pregnant and to use highly effective contraception while using AFINITOR and for up to 8 weeks after ending treatment [see Use in Specific Populations (8.6)].
Adverse Reactions
Clinical Trials Experience
- The efficacy and safety of AFINITOR (10 mg/day) plus exemestane (25 mg/day) (n=485) versus placebo plus exemestane (25 mg/day) (n=239) was evaluated in a randomized, controlled trial in patients with advanced or metastatic hormone receptor-positive, HER2-negative breast cancer. The median age of patients was 61 years (range 28-93 years), and 75% were Caucasian. Safety results are based on a median follow-up of approximately 13 months.
- The most common adverse reactions (incidence ≥ 30%) were stomatitis, infections, rash, fatigue, diarrhea, and decreased appetite. The most common Grade 3/4 adverse reactions (incidence ≥ 2%) were stomatitis, infections, hyperglycemia, fatigue, dyspnea, pneumonitis, and diarrhea. The most common laboratory abnormalities (incidence ≥ 50%) were hypercholesterolemia, hyperglycemia, increased aspartate transaminase (AST), anemia, leukopenia, thrombocytopenia, lymphopenia, increased alanine transaminase (ALT), and hypertriglyceridemia. The most common Grade 3/4 laboratory abnormalities (incidence ≥ 3%) were lymphopenia, hyperglycemia, anemia, decreased potassium, increased AST, increased ALT, and thrombocytopenia.
- Fatal adverse reactions occurred more frequently in patients who received AFINITOR plus exemestane (2%) compared to patients on the placebo plus exemestane arm (0.4%). The rates of treatment-emergent adverse events resulting in permanent discontinuation were 24% and 5% for the AFINITOR plus exemestane and placebo plus exemestane treatment groups, respectively. Dose adjustments (interruptions or reductions) were more frequent among patients in the AFINITOR plus exemestane arm than in the placebo plus exemestane arm (63% versus 14%).
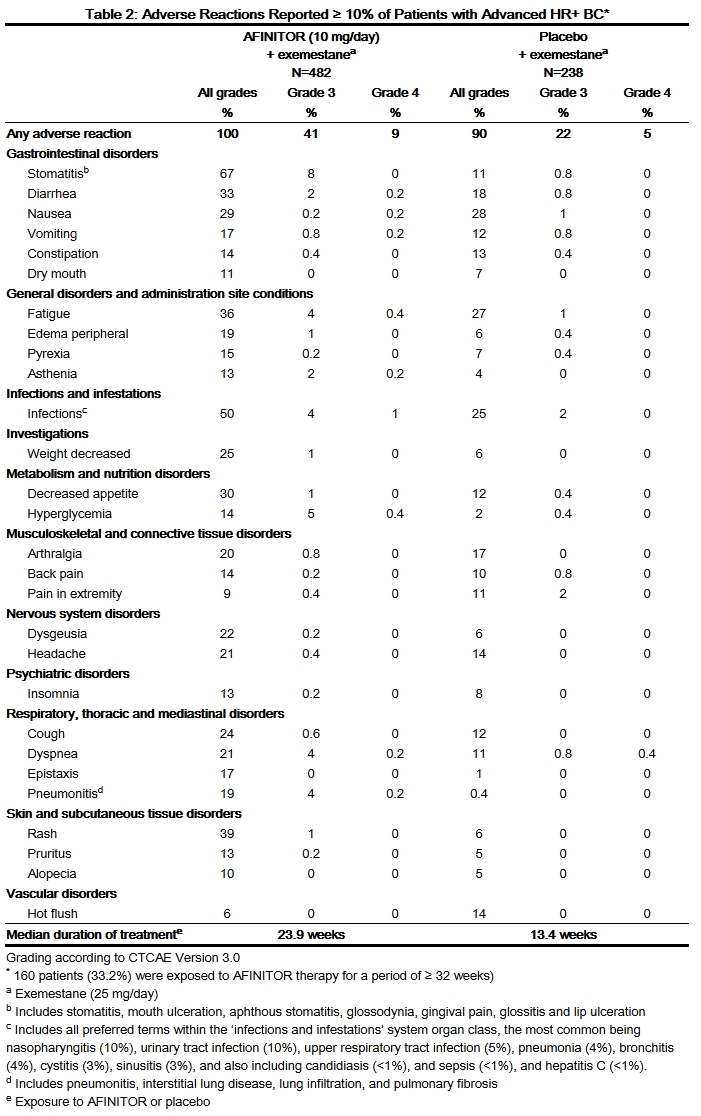
- Table 2 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥10% for patients receiving AFINITOR 10 mg daily versus placebo.
- Key observed laboratory abnormalities are presented in Table 3.
Clinical Study Experience in Advanced Pancreatic Neuroendocrine Tumors
- In a randomized, controlled trial of AFINITOR (n=204) versus placebo (n=203) in patients with advanced PNET the median age of patients was 58 years (range 20-87), 79% were Caucasian, and 55% were male. Patients on the placebo arm could cross over to open-label AFINITOR upon disease progression.
- The most common adverse reactions (incidence ≥ 30%) were stomatitis, rash, diarrhea, fatigue, edema, abdominal pain, nausea, fever, and headache. The most common Grade 3-4 adverse reactions (incidence ≥ 5%) were stomatitis and diarrhea. The most common laboratory abnormalities (incidence ≥ 50%) were decreased hemoglobin, hyperglycemia, alkaline phosphatase increased, hypercholesterolemia, bicarbonate decreased, and increased aspartate transaminase (AST). The most common Grade 3-4 laboratory abnormalities (incidence ≥ 3%) were hyperglycemia, lymphopenia, decreased hemoglobin, hypophosphatemia, increased alkaline phosphatase, neutropenia, increased aspartate transaminase (AST), potassium decreased, and thrombocytopenia. Deaths during double-blind treatment where an adverse event was the primary cause occurred in seven patients on AFINITOR and one patient on placebo. Causes of death on the AFINITOR arm included one case of each of the following: acute renal failure, acute respiratory distress, cardiac arrest, death (cause unknown), hepatic failure, pneumonia, and sepsis. There was one death due to pulmonary embolism on the placebo arm. After cross-over to open-label AFINITOR, there were three additional deaths, one due to hypoglycemia and cardiac arrest in a patient with insulinoma, one due to myocardial infarction with congestive heart failure, and the other due to sudden death. The rates of treatment-emergent adverse events resulting in permanent discontinuation were 20% and 6% for the AFINITOR and placebo treatment groups, respectively. Dose delay or reduction was necessary in 61% of everolimus patients and 29% of placebo patients. Grade 3-4 renal failure occurred in six patients in the everolimus arm and three patients in the placebo arm. Thrombotic events included five patients with pulmonary embolus in the everolimus arm and one in the placebo arm as well as three patients with thrombosis in the everolimus arm and two in the placebo arm.
- Table 4 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo.
- In female patients aged 18 to 55 years, irregular menstruation occurred in 5 of 46 (11%) AFINITOR-treated females and none of the 33 females in the placebo group.
- Key observed laboratory abnormalities are presented in Table 5.
Clinical Study Experience in Advanced Renal Cell Carcinoma
- The data described below reflect exposure to AFINITOR (n=274) and placebo (n=137) in a randomized, controlled trial in patients with metastatic renal cell carcinoma who received prior treatment with sunitinib and/or sorafenib. The median age of patients was 61 years (range 27-85), 88% were Caucasian, and 78% were male. The median duration of blinded study treatment was 141 days (range 19-451 days) for patients receiving AFINITOR and 60 days (range 21-295 days) for those receiving placebo.
- The most common adverse reactions (incidence ≥ 30%) were stomatitis, infections, asthenia, fatigue, cough, and diarrhea. The most common Grade 3-4 adverse reactions (incidence ≥ 3%) were infections, dyspnea, fatigue, stomatitis, dehydration, pneumonitis, abdominal pain, and asthenia. The most common laboratory abnormalities (incidence ≥ 50%) were anemia, hypercholesterolemia, hypertriglyceridemia, hyperglycemia, lymphopenia, and increased creatinine. The most common Grade 3-4 laboratory abnormalities (incidence ≥ 3%) were lymphopenia, hyperglycemia, anemia, hypophosphatemia, and hypercholesterolemia. Deaths due to acute respiratory failure (0.7%), infection (0.7%), and acute renal failure (0.4%) were observed on the AFINITOR arm but none on the placebo arm. The rates of treatment-emergent adverse events (irrespective of causality) resulting in permanent discontinuation were 14% and 3% for the AFINITOR and placebo treatment groups, respectively. The most common adverse reactions (irrespective of causality) leading to treatment discontinuation were pneumonitis and dyspnea. Infections, stomatitis, and pneumonitis were the most common reasons for treatment delay or dose reduction. The most common medical interventions required during AFINITOR treatment were for infections, anemia, and stomatitis.
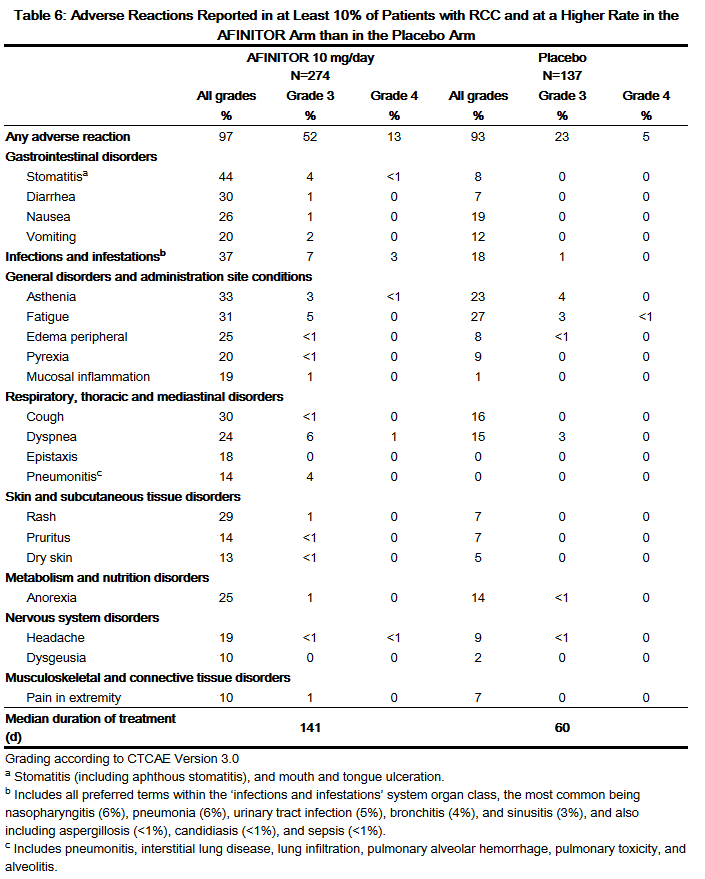
- Table 6 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo. Within each MedDRA system organ class, the adverse reactions are presented in order of decreasing frequency.
- Other notable adverse reactions occurring more frequently with AFINITOR than with placebo, but with an incidence of < 10% include:
- Gastrointestinal disorders: Abdominal pain (9%), dry mouth (8%), hemorrhoids (5%), dysphagia (4%)
- General disorders and administration site conditions: Weight decreased (9%), chest pain (5%), chills (4%), impaired wound healing (< 1%)
- Respiratory, thoracic and mediastinal disorders: Pleural effusion (7%), pharyngolaryngeal pain (4%), rhinorrhea (3%)
- Skin and subcutaneous tissue disorders: Hand-foot syndrome (reported as palmar-plantar erythrodysesthesia syndrome) (5%), nail disorder (5%), erythema (4%), onychoclasis (4%), skin lesion (4%), acneiform dermatitis (3%)
- Metabolism and nutrition disorders: Exacerbation of pre-existing diabetes mellitus (2%), new onset of diabetes mellitus (< 1%)
- Psychiatric disorders: Insomnia (9%)
- Nervous system disorders: Dizziness (7%), paresthesia (5%)
- Eye disorders: Eyelid edema (4%), conjunctivitis (2%)
- Vascular disorders: Hypertension (4%), deep vein thrombosis (< 1%)
- Renal and urinary disorders: Renal failure (3%)
- Cardiac disorders: Tachycardia (3%), congestive cardiac failure (1%)
- Musculoskeletal and connective tissue disorders: Jaw pain (3%)
- Hematologic disorders: Hemorrhage (3%)
- Key laboratory abnormalities are presented in Table 7.
Clinical Study Experience in Renal Angiomyolipoma with Tuberous Sclerosis Complex
- The data described below are based on a randomized (2:1), double-blind, placebo-controlled trial of AFINITOR in 118 patients with renal angiomyolipoma as a feature of TSC (n=113) or sporadic lymphangioleiomyomatosis (n=5). The median age of patients was 31 years (range 18 to 61 years), 89% were Caucasian, and 34% were male. The median duration of blinded study treatment was 48 weeks (range 2 to 115 weeks) for patients receiving AFINITOR and 45 weeks (range 9 to 115 weeks) for those receiving placebo.
- The most common adverse reaction reported for AFINITOR (incidence ≥ 30%) was stomatitis. The most common Grade 3-4 adverse reactions (incidence ≥ 2%) were stomatitis and amenorrhea. The most common laboratory abnormalities (incidence ≥ 50%) were hypercholesterolemia, hypertriglyceridemia, and anemia. The most common Grade 3-4 laboratory abnormality (incidence ≥ 3%) was hypophosphatemia.
- The rate of adverse reactions resulting in permanent discontinuation was 3.8% in the AFINITOR-treated patients. Adverse reactions leading to permanent discontinuation in the AFINITOR arm were hypersensitivity/angioedema/bronchospasm, convulsion, and hypophosphatemia. Dose adjustments (interruptions or reductions) due to adverse reactions occurred in 52% of AFINITOR-treated patients. The most common adverse reaction leading to AFINITOR dose adjustment was stomatitis.
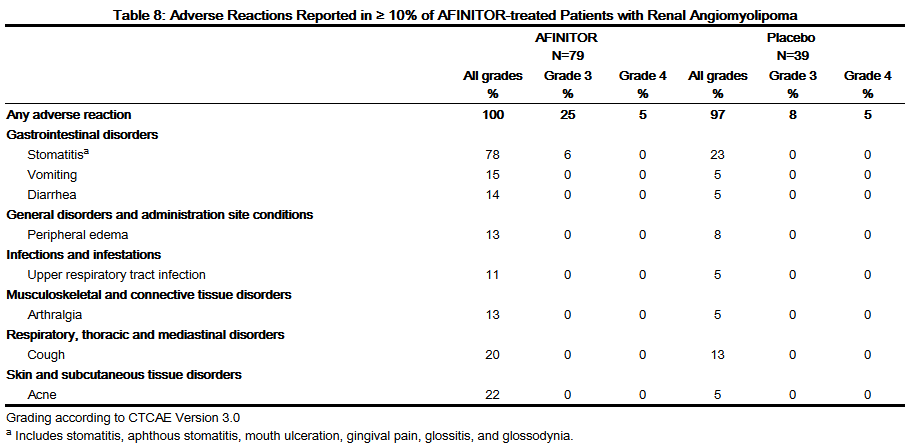
- Table 8 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 9.
- Amenorrhea occurred in 15% of AFINITOR-treated females (8 of 52) and 4% (1 of 26) of females in the placebo group. Other adverse reactions involving the female reproductive system were menorrhagia (10%), menstrual irregularities (10%), and vaginal hemorrhage (8%).
- The following additional adverse reactions occurred in less than 10% of Afinitor-treated patients: epistaxis (9%), decreased appetite (6%), otitis media (6%), depression (5%), abnormal taste (5%), increased blood luteinizing hormone (LH) levels (4%), increased blood follicle stimulating hormone (FSH) levels (3%), hypersensitivity (3%), and pneumonitis (1%).
Clinical Study Experience in Subependymal Giant Cell Astrocytoma with Tuberous Sclerosis Complex
- The data described below are based on a randomized (2:1), double-blind, placebo-controlled trial (Study 1) of AFINITOR in 117 patients with subependymal giant cell astrocytoma (SEGA) and tuberous sclerosis complex (TSC). The median age of patients was 9.5 years (range 0.8 to 26 years), 93% were Caucasian, and 57% were male. The median duration of blinded study treatment was 52 weeks (range 24 to 89 weeks) for patients receiving AFINITOR and 47 weeks (range 14 to 88 weeks) for those receiving placebo.
- The most common adverse reactions reported for AFINITOR (incidence ≥ 30%) were stomatitis and respiratory tract infection. The most common Grade 3-4 adverse reactions (incidence ≥ 2%) were stomatitis, pyrexia, pneumonia, gastroenteritis, aggression, agitation, and amenorrhea. The most common key laboratory abnormalities (incidence ≥ 50%) were hypercholesterolemia and elevated partial thromboplastin time. The most common Grade 3-4 laboratory abnormality (incidence ≥ 3%) was neutropenia.
- There were no adverse reactions resulting in permanent discontinuation. Dose adjustments (interruptions or reductions) due to adverse reactions occurred in 55% of AFINITOR-treated patients. The most common adverse reaction leading to AFINITOR dose adjustment was stomatitis.
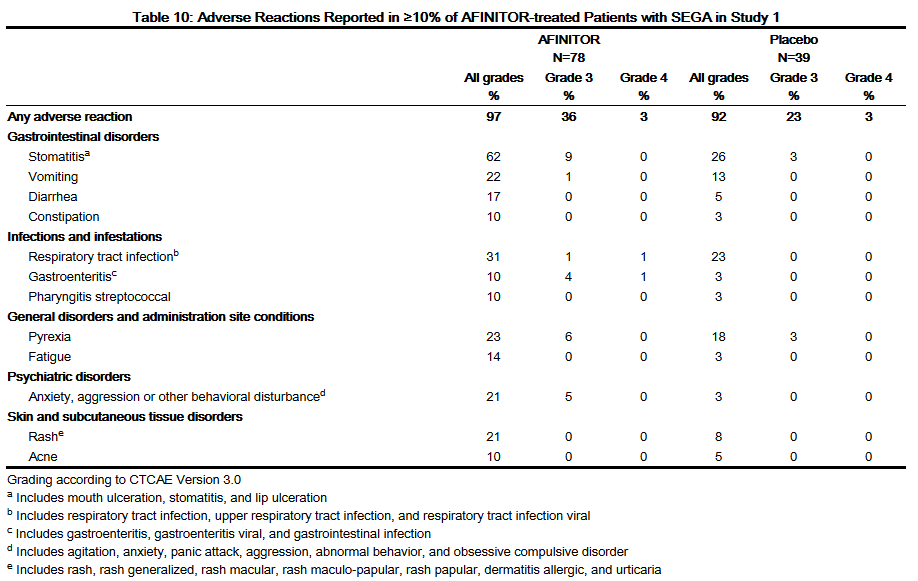
- Table 10 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 11.
- Amenorrhea occurred in 17% of AFINITOR-treated females aged 10 to 55 years (3 of 18) and none of the females in the placebo group. For this same group of AFINITOR-treated females, the following menstrual abnormalities were reported: dysmenorrhea (6%), menorrhagia (6%), metrorrhagia (6%), and unspecified menstrual irregularity (6%).
- The following additional adverse reactions occurred in less than 10% of AFINITOR-treated patients: nausea (8%), pain in extremity (8%), insomnia (6%), pneumonia (6%), epistaxis (5%), hypersensitivity (3%), increased blood luteinizing hormone (LH) levels (1%) and pneumonitis (1%).
- Longer-term follow-up of 34.2 months (range 4.7 to 47.1 months) from a non-randomized, open-label, 28-patient trial resulted in the following additional notable adverse reactions and key laboratory abnormalities: cellulitis (29%), hyperglycemia (25%), and elevated creatinine (14%).
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of AFINITOR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency or establish a causal relationship to drug exposure: acute pancreatitis, cholecystitis, cholelithiasis, arterial thrombotic events and reflex sympathetic dystrophy.
Drug Interactions
- Agents That May Increase Everolimus Blood Concentrations
- CYP3A4 Inhibitors and PgP Inhibitors
- In healthy subjects, compared to AFINITOR treatment alone there were significant increases in everolimus exposure when AFINITOR was coadministered with:
- ketoconazole (a strong CYP3A4 inhibitor and a PgP inhibitor) - Cmax and AUC increased by 3.9- and 15.0-fold, respectively.
- erythromycin (a moderate CYP3A4 inhibitor and a PgP inhibitor) - Cmax and AUC increased by 2.0- and 4.4-fold, respectively.
- verapamil (a moderate CYP3A4 inhibitor and a PgP inhibitor) - Cmax and AUC increased by 2.3- and 3.5-fold, respectively.
- In healthy subjects, compared to AFINITOR treatment alone there were significant increases in everolimus exposure when AFINITOR was coadministered with:
- CYP3A4 Inhibitors and PgP Inhibitors
- Concomitant strong inhibitors of CYP3A4/PgP should not be used [see Dosage and Administration (2.2, 2.5) and Warnings and Precautions (5.8)].
- Use caution when AFINITOR is used in combination with moderate CYP3A4/PgP inhibitors. If alternative treatment cannot be administered reduce the AFINITOR dose [see Dosage and Administration (2.2, 2.5) and Warnings and Precautions (5.8)].
- Agents That May Decrease Everolimus Blood Concentrations
- CYP3A4/PgP Inducers
- In healthy subjects, co-administration of AFINITOR with rifampin, a strong inducer of CYP3A4 and an inducer of PgP, decreased everolimus AUC and Cmax by 63% and 58% respectively, compared to everolimus treatment alone. Consider a dose increase of AFINITOR when co-administered with strong CYP3A4/PgP inducers if alternative treatment cannot be administered. St. John’s Wort may decrease everolimus exposure unpredictably and should be avoided [see Dosage and Administration (2.2, 2.5)].
- CYP3A4/PgP Inducers
- Drugs That May Have Their Plasma Concentrations Altered by Everolimus
- Studies in healthy subjects indicate that there are no clinically significant pharmacokinetic interactions between AFINITOR and the HMG-CoA reductase inhibitors atorvastatin (a CYP3A4 substrate) and pravastatin (a non-CYP3A4 substrate) and population pharmacokinetic analyses also detected no influence of simvastatin (a CYP3A4 substrate) on the clearance of AFINITOR.
- A study in healthy subjects demonstrated that co-administration of an oral dose of midazolam (sensitive CYP3A4 substrate) with everolimus resulted in a 25% increase in midazolam Cmax and a 30% increase in midazolam AUC(0-inf).
- Coadministration of everolimus and exemestane increased exemestane Cmin by 45% and C2h by 64%. However, the corresponding estradiol levels at steady state (4 weeks) were not different between the 2 treatment arms. No increase in adverse events related to exemestane was observed in patients with hormone receptor-positive, HER2-negative advanced breast cancer receiving the combination.
- Coadministration of everolimus and depot octreotide increased octreotide Cmin by approximately 50%.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Risk Summary
- Based on the mechanism of action, AFINITOR can cause fetal harm when administered to a pregnant woman. Everolimus caused embryo-fetal toxicities in animals at maternal exposures that were lower than human exposures. If this drug is used during pregnancy or if the patient becomes pregnant while taking the drug, apprise the patient of the potential hazard to the fetus [see Warnings and Precautions (5.11)].
- Animal Data
- In animal reproductive studies, oral administration of everolimus to female rats before mating and through organogenesis induced embryo-fetal toxicities, including increased resorption, pre-implantation and post-implantation loss, decreased numbers of live fetuses, malformation (e.g., sternal cleft), and retarded skeletal development. These effects occurred in the absence of maternal toxicities. Embryo-fetal toxicities in rats occurred at doses ≥ 0.1 mg/kg (0.6 mg/m2) with resulting exposures of approximately 4% of the exposure (AUC0-24h) achieved in patients receiving the 10 mg daily dose of everolimus. In rabbits, embryotoxicity evident as an increase in resorptions occurred at an oral dose of 0.8 mg/kg (9.6 mg/m2), approximately 1.6 times either the 10 mg daily dose or the median dose administered to SEGA patients on a body surface area basis. The effect in rabbits occurred in the presence of maternal toxicities.
- In a pre- and post-natal development study in rats, animals were dosed from implantation through lactation. At the dose of 0.1 mg/kg (0.6 mg/m2), there were no adverse effects on delivery and lactation or signs of maternal toxicity; however, there were reductions in body weight (up to 9% reduction from the control) and in survival of offspring (~5% died or missing). There were no drug-related effects on the developmental parameters (morphological development, motor activity, learning, or fertility assessment) in the offspring.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Everolimus in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Everolimus during labor and delivery.
Nursing Mothers
- It is not known whether everolimus is excreted in human milk. Everolimus and/or its metabolites passed into the milk of lactating rats at a concentration 3.5 times higher than in maternal serum. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from everolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Pediatric use of AFINITOR Tablets and AFINITOR DISPERZ is recommended for patients 1 year of age and older with TSC for the treatment of SEGA that requires therapeutic intervention but cannot be curatively resected. The safety and effectiveness of AFINITOR Tablets and AFINITOR DISPERZ have not been established in pediatric patients with renal angiomyolipoma with TSC in the absence of SEGA.
- The effectiveness of AFINITOR in pediatric patients with SEGA was demonstrated in two clinical trials based on demonstration of durable objective response, as evidenced by reduction in SEGA tumor volume [see Clinical Studies (14.5)]. Improvement in disease-related symptoms and overall survival in pediatric patients with SEGA has not been demonstrated. The long term effects of AFINITOR on growth and pubertal development are unknown.
- Study 1 was a randomized, double-blind, multicenter trial comparing AFINITOR (n=78) to placebo (n=39) in pediatric and adult patients. The median age was 9.5 years (range 0.8 to 26 years). At the time of randomization, a total of 20 patients were < 3 years of age, 54 patients were 3 to < 12 years of age, 27 patients were 12 to < 18 years of age, and 16 patients were ≥ 18 years of age. The overall nature, type, and frequency of adverse reactions across the age groups evaluated were similar, with the exception of a higher per patient incidence of infectious serious adverse events in patients < 3 years of age. A total of 6 of 13 patients (46%) < 3 years of age had at least 1 serious adverse event due to infection, compared to 2 of 7 patients (29%) treated with placebo. No patient in any age group discontinued AFINITOR due to infection [see Adverse Reactions (6.5)]. Subgroup analyses showed reduction in SEGA volume with AFINITOR treatment in all pediatric age subgroups.
- Study 2 was an open-label, single-arm, single-center trial of AFINITOR (N=28) in patients aged ≥ 3 years; median age was 11 years (range 3 to 34 years). A total of 16 patients were 3 to < 12 years, 6 patients were 12 to < 18 years, and 6 patients were ≥ 18 years. The frequency of adverse reactions across the age groups was generally similar [see Adverse Reactions (6.5)]. Subgroup analyses showed reductions in SEGA volume with AFINITOR treatment in all pediatric age subgroups.
- Everolimus clearance normalized to body surface area was higher in pediatric patients than in adults with SEGA [see Clinical Pharmacology (12.3)].The recommended starting dose and subsequent requirement for therapeutic drug monitoring to achieve and maintain trough concentrations of 5 to 15 ng/mL are the same for adult and pediatric patients with SEGA [see Dosage and Administration (2.3, 2.4)].
Geriatic Use
- In the randomized advanced hormone receptor positive, HER2-negative breast cancer study, 40% of AFINITOR-treated patients were ≥ 65 years of age, while 15% were 75 years and over. No overall differences in effectiveness were observed between elderly and younger patients. The incidence of deaths due to any cause within 28 days of the last AFINITOR dose was 6% in patients ≥ 65 years of age compared to 2% in patients < 65 years of age. Adverse reactions leading to permanent treatment discontinuation occurred in 33% of patients ≥ 65 years of age compared to 17% in patients < 65 years of age [see Warnings and Precautions (5.6)].
- In two other randomized trials (advanced renal cell carcinoma and advanced neuroendocrine tumors of pancreatic origin), no overall differences in safety or effectiveness were observed between elderly and younger patients. In the randomized advanced RCC study, 41% of AFINITOR treated patients were ≥ 65 years of age, while 7% were 75 years and over. In the randomized advanced PNET study, 30% of AFINITOR-treated patients were ≥ 65 years of age, while 7% were 75 years and over.
- Other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)].
- No dosage adjustment in initial dosing is required in elderly patients, but close monitoring and appropriate dose adjustments for adverse reactions is recommended [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
Gender
There is no FDA guidance on the use of Everolimus with respect to specific gender populations.
Race
There is no FDA guidance on the use of Everolimus with respect to specific racial populations.
Renal Impairment
- No clinical studies were conducted with AFINITOR in patients with decreased renal function. Renal impairment is not expected to influence drug exposure and no dosage adjustment of everolimus is recommended in patients with renal impairment [see Clinical Pharmacology (12.3)].
Hepatic Impairment
- The safety, tolerability and pharmacokinetics of AFINITOR were evaluated in a 34 subject single oral dose study of everolimus in subjects with impaired hepatic function relative to subjects with normal hepatic function. Exposure was increased in patients with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe (Child-Pugh class C) hepatic impairment [see Clinical Pharmacology (12.3)].
- For advanced HR+ BC, advanced PNET, advanced RCC, and renal angiomyolipoma with TSC patients with severe hepatic impairment, AFINITOR may be used at a reduced dose if the desired benefit outweighs the risk. For patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, a dose reduction is recommended [see Dosage and Administration (2.2)].
- For patients with SEGA who have severe hepatic impairment (Child-Pugh class C), reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50%. For patients with SEGA who have mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, adjustment to the starting dose may not be needed. Subsequent dosing should be based on therapeutic drug monitoring [see Dosage and Administration (2.4, 2.5)].
Females of Reproductive Potential and Males
- Contraception
- Females
- AFINITOR can cause fetal harm when administered to a pregnant woman. Advise female patients of reproductive potential to use highly effective contraception while receiving AFINITOR and for up to 8 weeks after ending treatment [see Use in Specific Populations (8.1)].
- Females
- Infertility
- Females
- Menstrual irregularities, secondary amenorrhea, and increases in luteinizing hormone (LH) and follicle stimulating hormone (FSH) occurred in female patients taking AFINITOR. Based on these clinical findings and findings in animals, female fertility may be compromised by treatment with AFINITOR [see Adverse Reactions (6.2, 6.4, 6.5) and Nonclinical Toxicology (13.1)].
- Females
- Males
- AFINITOR treatment may impair fertility in male patients based on animal findings [see Nonclinical Toxicology (13.1)].
- Males
Immunocompromised Patients
There is no FDA guidance one the use of Everolimus in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Renal function of patients should be monitored particularly where patients have additional risk factors that may further impair renal function.
- Monitoring of fasting serum glucose and lipid profile is recommended prior to the start of AFINITOR therapy and periodically thereafter as well as management with appropriate medical therapy.
- Monitoring of complete blood count is recommended prior to the start of AFINITOR therapy and periodically thereafter.
IV Compatibility
There is limited information regarding IV Compatibility of Everolimus in the drug label.
Overdosage
Acute Overdose
In animal studies, everolimus showed a low acute toxic potential. No lethality or severe toxicity was observed in either mice or rats given single oral doses of 2000 mg/kg (limit test).
Reported experience with overdose in humans is very limited. Single doses of up to 70 mg have been administered. The acute toxicity profile observed with the 70 mg dose was consistent with that for the 10 mg dose.
Chronic Overdose
There is limited information regarding Chronic Overdose of Everolimus in the drug label.
Pharmacology
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | 42-O-(2-hydroxyethyl)rapamycin |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | ~30 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C53H83NO14 |
| Molar mass | 958.224 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Mechanism of Action
- Everolimus is an inhibitor of mammalian target of rapamycin (mTOR), a serine-threonine kinase, downstream of the PI3K/AKT pathway. The mTOR pathway is dysregulated in several human cancers. Everolimus binds to an intracellular protein, FKBP-12, resulting in an inhibitory complex formation with mTOR complex 1 (mTORC1) and thus inhibition of mTOR kinase activity. Everolimus reduced the activity of S6 ribosomal protein kinase (S6K1) and eukaryotic elongation factor 4E-binding protein (4E-BP1), downstream effectors of mTOR, involved in protein synthesis. S6K1 is a substrate of mTORC1 and phosphorylates the activation domain 1 of the estrogen receptor which results in ligand-independent activation of the receptor. In addition, everolimus inhibited the expression of hypoxia-inducible factor (e.g., HIF-1) and reduced the expression of vascular endothelial growth factor (VEGF). Inhibition of mTOR by everolimus has been shown to reduce cell proliferation, angiogenesis, and glucose uptake in in vitro and/or in vivo studies.
- Constitutive activation of the PI3K/Akt/mTOR pathway can contribute to endocrine resistance in breast cancer. In vitro studies show that estrogen-dependent and HER2+ breast cancer cells are sensitive to the inhibitory effects of everolimus, and that combination treatment with everolimus and Akt, HER2, or aromatase inhibitors enhances the anti-tumor activity of everolimus in a synergistic manner.
- Two regulators of mTORC1 signaling are the oncogene suppressors tuberin-sclerosis complexes 1 and 2 (TSC1, TSC2). Loss or inactivation of either TSC1 or TSC2 leads to activation of downstream signaling. In TSC, a genetic disorder, inactivating mutations in either the TSC1 or the TSC2 gene lead to hamartoma formation throughout the body.
Structure
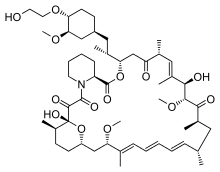
- AFINITOR (everolimus), an inhibitor of mammalian target of rapamycin (mTOR), is an antineoplastic agent.
- The chemical name of everolimus is (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18- dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone.
- The molecular formula is C53H83NO14 and the molecular weight is 958.2. The structural formula is:
- AFINITOR Tablets are supplied for oral administration and contain 2.5 mg, 5 mg, 7.5 mg, or 10 mg of everolimus. The tablets also contain anhydrous lactose, butylated hydroxytoluene, crospovidone, hypromellose, lactose monohydrate, and magnesium stearate as inactive ingredients.
- AFINITOR DISPERZ (everolimus tablets for oral suspension) is supplied for oral administration and contains 2 mg, 3 mg, or 5 mg of everolimus. The tablets for oral suspension also contain butylated hydroxytoluene, colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, mannitol, and microcrystalline cellulose as inactive ingredients.
Pharmacodynamics
- Exposure Response Relationships
- Markers of protein synthesis show that inhibition of mTOR is complete after a 10 mg daily dose.
- In patients with SEGA, higher everolimus trough concentrations appear to be associated with larger reductions in SEGA volume. However, as responses have been observed at trough concentrations as low as 5 ng/mL, once acceptable efficacy has been achieved, additional dose increase may not be necessary.
Pharmacokinetics
- Absorption
- After administration of AFINITOR tablets in patients with advanced solid tumors, peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses ranging from 5 mg to 70 mg. Following single doses, Cmax is dose-proportional with daily dosing between 5 mg and 10 mg. With single doses of 20 mg and higher, the increase in Cmax is less than dose-proportional, however AUC shows dose-proportionality over the 5 mg to 70 mg dose range. Steady-state was achieved within 2 weeks following once-daily dosing.
- Dose Proportionality in Patients with SEGA and TSC: In patients with SEGA and TSC, everolimus Cmin was approximately dose-proportional within the dose range from 1.35 mg/m2 to 14.4 mg/m2.
- Food effect: In healthy subjects, high-fat meals reduced systemic exposure to AFINITOR 10 mg tablet (as measured by AUC) by 22% and the peak blood concentration Cmax by 54%. Light-fat meals reduced AUC by 32% and Cmax by 42%. Food, however, had no apparent effect on the post absorption phase concentration-time profile.
- Relative bioavailability of AFINITOR DISPERZ (everolimus tablets for oral suspension): The AUC0-∞ of AFINITOR DISPERZ was equivalent to that of AFINITOR Tablets; the Cmax of this dosage form was 20%-36% lower than that of AFINITOR Tablets. The predicted trough concentrations at steady-state were similar after daily administration.
- Distribution
- The blood-to-plasma ratio of everolimus, which is concentration-dependent over the range of 5 to 5000 ng/mL, is 17% to 73%. The amount of everolimus confined to the plasma is approximately 20% at blood concentrations observed in cancer patients given AFINITOR 10 mg/day. Plasma protein binding is approximately 74% both in healthy subjects and in patients with moderate hepatic impairment.
- Metabolism
- Everolimus is a substrate of CYP3A4 and PgP. Following oral administration, everolimus is the main circulating component in human blood. Six main metabolites of everolimus have been detected in human blood, including three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidylcholine conjugate of everolimus. These metabolites were also identified in animal species used in toxicity studies, and showed approximately 100-times less activity than everolimus itself.
- In vitro, everolimus competitively inhibited the metabolism of CYP3A4 and was a mixed inhibitor of the CYP2D6 substrate dextromethorphan.
- Elimination
- No specific elimination studies have been undertaken in cancer patients. Following the administration of a 3 mg single dose of radiolabeled everolimus in patients who were receiving cyclosporine, 80% of the radioactivity was recovered from the feces, while 5% was excreted in the urine. The parent substance was not detected in urine or feces. The mean elimination half-life of everolimus is approximately 30 hours.
- Patients with Renal Impairment
- Approximately 5% of total radioactivity was excreted in the urine following a 3 mg dose of [14C]-labeled everolimus. In a population pharmacokinetic analysis which included 170 patients with advanced cancer, no significant influence of creatinine clearance (25–178 mL/min) was detected on oral clearance (CL/F) of everolimus [see Use in Specific Populations (8.7)].
- Patients with Hepatic Impairment
- The safety, tolerability and pharmacokinetics of AFINITOR were evaluated in a single oral dose study of everolimus in subjects with impaired hepatic function relative to subjects with normal hepatic function. Compared to normal subjects (N=13), there was a 1.8-fold, 3.2-fold, and 3.6-fold increase in exposure (i.e. AUC) for subjects with mild (Child-Pugh class A, n=6), moderate (Child-Pugh class B, n=9), and severe (Child-Pugh class C, n=6) hepatic impairment, respectively. In another study, the average AUC of everolimus in eight subjects with moderate hepatic impairment (Child-Pugh class B) was twice that found in eight subjects with normal hepatic function.
- For advanced HR+ BC, advanced PNET, advanced RCC, and renal angiomyolipoma with TSC patients with severe hepatic impairment, AFINITOR may be used at a reduced dose if the desired benefit outweighs the risk. For patients with moderate or mild hepatic impairment, a dose reduction is recommended [see Dosage and Administration (2.2)].
- For patients with SEGA and mild or moderate hepatic impairment, adjust the dose of AFINITOR Tablets or AFINITOR DISPERZ based on therapeutic drug monitoring. For patients with SEGA and severe hepatic impairment, reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50% and adjust subsequent doses based on therapeutic drug monitoring [see Dosage and Administration (2.4, 2.5)].
- Effects of Age and Gender
- In a population pharmacokinetic evaluation in cancer patients, no relationship was apparent between oral clearance and patient age or gender.
- In patients with SEGA, the geometric mean Cmin values normalized to mg/m2 dose in patients aged < 10 years and 10 to 18 years were lower by 54% and 40%, respectively, than those observed in adults (> 18 years of age), suggesting that everolimus clearance normalized to body surface area was higher in pediatric patients as compared to adults.
- Ethnicity
- Based on a cross-study comparison, Japanese patients (n=6) had on average exposures that were higher than non-Japanese patients receiving the same dose.
- Based on analysis of population pharmacokinetics, oral clearance (CL/F) is on average 20% higher in black patients than in Caucasians.
- The significance of these differences on the safety and efficacy of everolimus in Japanese or black patients has not been established.
- QT/QTc Prolongation Potential
- In a randomized, placebo-controlled, cross-over study, 59 healthy subjects were administered a single oral dose of AFINITOR (20 mg and 50 mg) and placebo. There is no indication of a QT/QTc prolonging effect of AFINITOR in single doses up to 50 mg.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Administration of everolimus for up to 2 years did not indicate oncogenic potential in mice and rats up to the highest doses tested (0.9 mg/kg) corresponding respectively to 3.9 and 0.2 times the estimated clinical exposure (AUC0-24h) at the 10 mg daily human dose.
- Everolimus was not genotoxic in a battery of in vitro assays (Ames mutation test in Salmonella, mutation test in L5178Y mouse lymphoma cells, and chromosome aberration assay in V79 Chinese hamster cells). Everolimus was not genotoxic in an in vivo mouse bone marrow micronucleus test at doses up to 500 mg/kg/day (1500 mg/m2/day, approximately 255-fold the 10 mg daily human dose, and 103-fold the maximum dose administered to patients with SEGA, based on the body surface area), administered as 2 doses, 24 hours apart.
- Based on non-clinical findings, male fertility may be compromised by treatment with AFINITOR. In a 13-week male fertility study in rats, testicular morphology was affected at 0.5 mg/kg and above. Sperm motility, sperm count, and plasma testosterone levels were diminished in rats treated with 5 mg/kg. These doses result in exposures which are within the range of therapeutic exposure (52 ng•hr/mL and 414 ng•hr/mL respectively compared to 560 ng•hr/mL human exposure at 10 mg/day), and resulted in infertility in the rats at 5 mg/kg. Effects on male fertility occurred at the AUC0-24h values below that of therapeutic exposure (approximately 10%-81% of the AUC0-24h in patients receiving the 10 mg daily dose). After a 10-13 week non-treatment period, the fertility index increased from zero (infertility) to 60% (12/20 mated females were pregnant).
- Oral doses of everolimus in female rats at ≥0.1 mg/kg (approximately 4% the AUC0-24h in patients receiving the 10 mg daily dose) resulted in increased incidence of pre-implantation loss, suggesting that the drug may reduce female fertility.
Animal Toxicology and/or Pharmacology
- In juvenile rat toxicity studies, dose-related delayed attainment of developmental landmarks including delayed eye-opening, delayed reproductive development in males and females and increased latency time during the learning and memory phases were observed at doses as low as 0.15 mg/kg/day.
Clinical Studies
Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer
- A randomized, double-blind, multicenter study of AFINITOR plus exemestane versus placebo plus exemestane was conducted in 724 postmenopausal women with estrogen receptor-positive, HER 2/neu-negative advanced breast cancer with recurrence or progression following prior therapy with letrozole or anastrozole. Randomization was stratified by documented sensitivity to prior hormonal therapy (yes versus no) and by the presence of visceral metastasis (yes versus no). Sensitivity to prior hormonal therapy was defined as either (1) documented clinical benefit (complete response [CR], partial response [PR], stable disease ≥ 24 weeks) to at least one prior hormonal therapy in the advanced setting or (2) at least 24 months of adjuvant hormonal therapy prior to recurrence. Patients were permitted to have received 0-1 prior lines of chemotherapy for advanced disease.
- The primary endpoint for the trial was progression-free survival (PFS) evaluated by Response Evaluation Criteria In Solid Tumors (RECIST), based on investigator (local radiology) assessment. Other endpoints included overall survival (OS), objective response rate (ORR), and safety.
- Patients were randomly allocated in a 2:1 ratio to AFINITOR 10 mg/day plus exemestane 25 mg/day (n = 485) or to placebo plus exemestane 25 mg/day (n = 239). The two treatment groups were generally balanced with respect to baseline demographics and disease characteristics. Patients were not permitted to cross over to AFINITOR at the time of disease progression.
- The median progression-free survival by investigator assessment at the time of the final PFS analysis was 7.8 and 3.2 months in the AFINITOR and placebo arms, respectively [HR = 0.45 (95% CI: 0.38, 0.54), one-sided log-rank p < 0.0001] (see Table 12 and Figure 1). The results of the PFS analysis based on independent central radiological assessment were consistent with the investigator assessment. PFS results were also consistent across the subgroups of age, race, presence and extent of visceral metastases, and sensitivity to prior hormonal therapy.
- Objective response rate was 12.6% (95% CI: 9.8, 15.9) in the AFINITOR plus exemestane arm versus 1.7% (95% CI: 0.5, 4.2) in the placebo plus exemestane arm. There were 3 complete responses (0.6%) and 58 partial responses (12.0%) in the AFINITOR plus exemestane arm. There were no complete responses and 4 partial responses (1.7%) in the placebo plus exemestane arm.
- The overall survival results were not mature at the time of the interim analysis, and no statistically significant treatment-related difference in OS was noted [HR=0.77 (95% CI: 0.57, 1.04)].
Advanced Neuroendocrine Tumors
Locally Advanced or Metastatic Advanced Pancreatic Neuroendocrine Tumors (PNET)
- A randomized, double-blind, multi-center trial of AFINITOR plus best supportive care (BSC) versus placebo plus BSC was conducted in patients with locally advanced or metastatic advanced pancreatic neuroendocrine tumors (PNET) and disease progression within the prior 12 months. Patients were stratified by prior cytotoxic chemotherapy (yes versus no) and by WHO performance status (0 versus 1 and 2). Treatment with somatostatin analogs was allowed as part of BSC. The primary endpoint for the trial was progression-free survival (PFS) evaluated by RECIST (Response Evaluation Criteria in Solid Tumors). After documented radiological progression, patients could be unblinded by the investigator; those randomized to placebo were then able to receive open-label AFINITOR. Other endpoints included safety, objective response rate [ORR (complete response (CR) or partial response (PR)], response duration, and overall survival.
- Patients were randomized 1:1 to receive either AFINITOR 10 mg/day (n=207) or placebo (n=203). Demographics were well balanced (median age 58 years, 55% male, 79% Caucasian). Cross-over from placebo to open-label AFINITOR occurred in 73% (148/203) of patients.
- The trial demonstrated a statistically significant improvement in PFS (median 11.0 months versus 4.6 months), resulting in a 65% risk reduction in investigator-determined PFS (HR 0.35; 95%CI: 0.27 to 0.45; p<0.001) (see Table 13 and Figure 2). PFS improvement was observed across all patient subgroups, irrespective of prior somatostatin analog use. The PFS results by investigator radiological review, central radiological review and adjudicated radiological review are shown below in Table 13.
- Investigator-determined response rate was low (4.8%) in the AFINITOR arm and there were no complete responses. The overall survival results are not yet mature and no statistically significant treatment-related difference in OS was noted [HR=1.05 (95% CI: 0.71 to 1.55)].
- Lack of Efficacy in Locally Advanced or Metastatic Functional Carcinoid Tumors
- The safety and effectiveness of AFINITOR in patients with locally advanced or metastatic functional carcinoid tumors have not been demonstrated. In a randomized (1:1), double-blind, multi-center trial in 429 patients with carcinoid tumors, AFINITOR plus depot octreotide (Sandostatin LAR®) was compared to placebo plus depot octreotide. After documented radiological progression, patients on the placebo arm could receive AFINITOR; of those randomized to placebo, 143 (67%) patients received open-label AFINITOR plus depot octreotide. The study did not meet its primary efficacy endpoint of a statistically significant improvement in PFS and the final analysis of OS favored the placebo plus depot octreotide arm.
Advanced Renal Cell Carcinoma
- An international, multi-center, randomized, double-blind trial comparing AFINITOR 10 mg daily and placebo, both in conjunction with best supportive care, was conducted in patients with metastatic RCC whose disease had progressed despite prior treatment with sunitinib, sorafenib, or both sequentially. Prior therapy with bevacizumab, interleukin 2, or interferon-α was also permitted. Randomization was stratified according to prognostic score1 and prior anticancer therapy.
- Progression-free survival (PFS), documented using Response Evaluation Criteria in Solid Tumors (RECIST) was assessed via a blinded, independent, central radiologic review. After documented radiological progression, patients could be unblinded by the investigator: those randomized to placebo were then able to receive open-label AFINITOR 10 mg daily.
- In total, 416 patients were randomized 2:1 to receive AFINITOR (n=277) or placebo (n=139). Demographics were well balanced between the 2 arms (median age 61 years; 77% male, 88% Caucasian, 74% received prior sunitinib or sorafenib, and 26% received both sequentially).
- AFINITOR was superior to placebo for PFS (see Table 14 and Figure 3). The treatment effect was similar across prognostic scores and prior sorafenib and/or sunitinib. Final overall survival (OS) results yield a hazard ratio of 0.90 (95% CI: 0.71 to 1.14), with no statistically significant difference between the 2 treatment groups. Planned cross-over from placebo due to disease progression to open label AFINITOR occurred in 111 of the 139 patients (79.9%) and may have confounded the OS benefit.
Renal Angiomyolipoma with Tuberous Sclerosis Complex
- A randomized (2:1), double-blind, placebo-controlled trial of AFINITOR was conducted in 118 patients with renal angiomyolipoma as a feature of TSC (n=113) or sporadic lymphangioleiomyomatosis (n=5).
- The key eligibility requirements for this trial were at least one angiomyolipoma of ≥ 3 cm in longest diameter on CT/MRI based on local radiology assessment, no immediate indication for surgery, and age ≥ 18 years. Patients received daily oral AFINITOR 10 mg or matching placebo until disease progression or unacceptable toxicity. CT or MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks and annually thereafter. Clinical and photographic assessment of skin lesions were conducted at baseline and every 12 weeks thereafter until treatment discontinuation. The major efficacy outcome measure was angiomyolipoma response rate based on independent central radiology review, which was defined as a ≥ 50% reduction in angiomyolipoma volume, absence of new angiomyolipoma lesion ≥ 1 cm, absence of kidney volume increase ≥ 20%, and no angiomyolipoma related bleeding of ≥ Grade 2. Key supportive efficacy outcome measures were time to angiomyolipoma progression and skin lesion response rate. Analyses of efficacy outcome measures were limited to the blinded treatment period which ended 6 months after the last patient was randomized. The comparative angiomyolipoma response rate analysis was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes versus no).
- Of the 118 patients enrolled, 79 were randomized to AFINITOR and 39 to placebo. The median age was 31 years (range 18 to 61 years), 34% were male, and 89% were Caucasian. At baseline, 17% of patients were receiving EIAEDs. On central radiology review at baseline, 92% of patients had at least 1 angiomyolipoma of ≥ 3 cm in longest diameter, 29% had angiomyolipomas ≥ 8 cm, 78% had bilateral angiomyolipomas, and 97% had skin lesions. The median values for the sum of all target renal angiomyolipoma lesions at baseline were 85 cm3 (range 9 to 1612 cm3) and 120 cm3 (range 3 to 4520 cm3) in the AFINITOR and placebo arms respectively. Forty-six (39%) patients had prior renal embolization or nephrectomy. The median duration of follow-up was 8.3 months (range 0.7 to 24.8 months).
- The renal angiomyolipoma response rate was statistically significantly higher in AFINITOR-treated patients; there were 33 (41.8%) patients with angiomyolipoma responses in the AFINITOR arm as compared to none in the placebo arm. Results are displayed in Table 15. The median response duration was 5.3+ months (range 2.3+ to 19.6+ months).
- There were 3 patients in the AFINITOR arm and 8 patients in the placebo arm with documented angiomyolipoma progression by central radiologic review. The time to angiomyolipoma progression was statistically significantly longer in the AFINITOR arm (HR 0.08 [95% CI: 0.02, 0.37]; p <0.0001).
- Skin lesion response rates were assessed by local investigators in 77 patients in the AFINITOR arm and 37 patients in the placebo arm with skin lesions at study entry. The skin lesion response rate was statistically significantly higher in the AFINITOR arm (26% versus 0, p=0.0011); all skin lesion responses were partial responses, defined as visual improvement in 50%-99% skin lesions, considering all skin lesions, durable for at least 8 weeks (Physician's Global Assessment of Clinical Condition).
Subependymal Giant Cell Astrocytoma with Tuberous Sclerosis Complex
- Study 1 was a randomized (2:1), double-blind, placebo-controlled trial of AFINITOR Tablets conducted in 117 pediatric and adult patients with subependymal giant cell astrocytoma (SEGA) and tuberous sclerosis complex (TSC). Eligible patients had at least one SEGA lesion ≥ 1.0 cm in longest diameter on MRI based on local radiology assessment and one or more of the following: serial radiological evidence of SEGA growth, a new SEGA lesion ≥ 1 cm in longest diameter, or new or worsening hydrocephalus. Patients randomized to the treatment arm received AFINITOR Tablets at a starting dose of 4.5 mg/m2 daily, with subsequent dose adjustments as needed to achieve and maintain everolimus trough concentrations of 5 to 15 ng/mL as tolerated. AFINITOR/matched placebo treatment continued until disease progression or unacceptable toxicity. MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks, and annually thereafter.
- The main efficacy outcome measure was SEGA response rate based on independent central radiology review. SEGA response was defined as a ≥ 50% reduction in the sum of SEGA volume relative to baseline, in the absence of unequivocal worsening of non-target SEGA lesions, a new SEGA lesion ≥ 1 cm, and new or worsening hydrocephalus. Analysis of SEGA response rate was limited to the blinded treatment period which ended 6 months after the last patient was randomized. The analysis of SEGA response rate was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes versus no).
- Of the 117 patients enrolled, 78 were randomized to AFINITOR and 39 to placebo. The median age was 9.5 years (range 0.8 to 26 years; 69% were 3 to < 18 years at enrollment; 17% were < 3 years at enrollment), 57% were male, and 93% were Caucasian. At baseline, 18% of patients were receiving EIAEDs. Based on central radiology review at baseline, 98% of patients had at least one SEGA lesion ≥ 1.0 cm in longest diameter, 79% had bilateral SEGAs, 43% had ≥ 2 target SEGA lesions, 26% had growth in or into the inferior surface of the ventricle, 9% had evidence of growth beyond the subependymal tissue adjacent to the ventricle, and 7% had radiographic evidence of hydrocephalus. The median values for the sum of all target SEGA lesions at baseline were 1.63 cm3 (range 0.18 to 25.15 cm3) and 1.30 cm3 (range 0.32 to 9.75 cm3) in the AFINITOR and placebo arms respectively. Eight (7%) patients had prior SEGA-related surgery. The median duration of follow-up was 8.4 months (range 4.6 to 17.2 months).
- The SEGA response rate was statistically significantly higher in AFINITOR-treated patients. There were 27 (35%) patients with SEGA responses in the AFINITOR arm and no SEGA responses in the placebo arm. Results are displayed in Table 16. At the time of the final analysis, all SEGA responses were ongoing and the median duration of response was 5.3 months (range 2.1 to 8.4 months). No patient in either treatment arm required surgical intervention during the course of Study 1.
- With a median follow-up of 8.4 months, SEGA progression was detected in 6 of 39 (15.4%) patients randomized to receive placebo and none of the 78 patients randomized to receive AFINITOR.
- Study 2 was an open-label, single-arm trial conducted to evaluate the safety and efficacy of AFINITOR in patients with SEGA and TSC. Serial radiological evidence of SEGA growth was required for entry. Change in SEGA volume at the end of the core 6-month treatment phase was assessed via independent central radiology review. In total, 28 patients received treatment with AFINITOR; median age was 11 years (range 3-34), 61% male, 86% Caucasian. Four patients had surgical resection of their SEGA lesions with subsequent re-growth prior to receiving AFINITOR treatment. After the core treatment phase, patients could continue to receive AFINITOR treatment as part of an extension treatment phase where SEGA volume was assessed every 6 months. The median duration of treatment was 34.2 months (range 4.7-47.1 months).
- At 6 months, nine of 28 patients (32%, 95% CI: 16% to 52%) had a ≥ 50% reduction in the tumor volume of their largest SEGA lesion. The median duration of response for these nine patients was 11.8 months (range 3.2 to 39.1 months). Seven of these nine patients had an ongoing volumetric reduction of ≥ 50% at the data cutoff.
- Three of four patients who had prior surgery experienced a ≥ 50% reduction in the tumor volume of their largest SEGA lesion. One of these three patients responded by month 6. No patient developed new lesions.
How Supplied
AFINITOR (everolimus) Tablets
- 2.5 mg tablets
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “LCL” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0594-51
- Each carton contains 4 blister cards of 7 tablets each
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “LCL” on one side and “NVR” on the other; available in:
- 5 mg tablets
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “5” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0566-51
- Each carton contains 4 blister cards of 7 tablets each
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “5” on one side and “NVR” on the other; available in:
- 7.5 mg tablets
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “7P5” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0620-51
- Each carton contains 4 blister cards of 7 tablets each
- 10 mg tablets
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “UHE” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0567-51
- Each carton contains 4 blister cards of 7 tablets each
- White to slightly yellow, elongated tablets with a bevelled edge and no score, engraved with “UHE” on one side and “NVR” on the other; available in:
AFINITOR DISPERZ (everolimus tablets for oral suspension)
- 2 mg tablets for oral suspension
- White to slightly yellowish, round, flat tablets with a bevelled edge and no score, engraved with “D2” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0626-51
- Each carton contains 4 blister cards of 7 tablets each
- White to slightly yellowish, round, flat tablets with a bevelled edge and no score, engraved with “D2” on one side and “NVR” on the other; available in:
- 3 mg tablets for oral suspension
- White to slightly yellowish, round, flat tablets with a bevelled edge and no score, engraved with “D3” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0627-51
- Each carton contains 4 blister cards of 7 tablets each
- White to slightly yellowish, round, flat tablets with a bevelled edge and no score, engraved with “D3” on one side and “NVR” on the other; available in:
- 5 mg tablets for oral suspension
- White to slightly yellowish, round, flat tablets with a bevelled edge and no score, engraved with “D5” on one side and “NVR” on the other; available in:
- Blisters of 28 tablets………………………………………………………………………………NDC 0078-0628-51
- Each carton contains 4 blister cards of 7 tablets each
- White to slightly yellowish, round, flat tablets with a bevelled edge and no score, engraved with “D5” on one side and “NVR” on the other; available in:
- Store AFINITOR (everolimus) Tablets and AFINITOR DISPERZ (everolimus tablets for oral suspension) at 25°C (77°F); excursions permitted between 15°–30°C (59°–86°F). See USP Controlled Room Temperature. Store in the original container, protect from light and moisture. Keep this and all drugs out of the reach of children.
- Follow special handling and disposal procedures for anticancer pharmaceuticals.
- AFINITOR Tablets and AFINITOR DISPERZ should not be crushed. Do not take tablets which are crushed or broken.
Storage
There is limited information regarding Everolimus Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Everolimus |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Everolimus |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
- Non-infectious Pneumonitis
- Warn patients of the possibility of developing non-infectious pneumonitis. In clinical studies, some non-infectious pneumonitis cases have been severe and occasionally fatal. Advise patients to report promptly any new or worsening respiratory symptoms [see Warnings and Precautions (5.1)].
- Infections
- Inform patients that they are more susceptible to infections while being treated with AFINITOR and that cases of hepatitis B reactivation have been associated with AFINITOR treatment. In clinical studies, some of these infections have been severe (e.g., leading to sepsis, respiratory or hepatic failure) and occasionally fatal. Patients should be aware of the signs and symptoms of infection and should report any such signs or symptoms promptly to their physician [see Warnings and Precautions (5.2)].
- Oral Ulceration
- Inform patients of the possibility of developing mouth ulcers, stomatitis, and oral mucositis. In such cases, mouthwashes and/or topical treatments are recommended, but these should not contain alcohol, peroxide, iodine, or thyme [see Warnings and Precautions (5.3)].
- Renal Failure
- Inform patients of the possibility of developing kidney failure. In some cases kidney failure has been severe and occasionally fatal. Inform patients of the need for the healthcare provider to monitor kidney function, especially in patients with risk factors that may impair kidney function [see Warnings and Precautions (5.4)].
- Impaired Wound Healing
- Inform patients of the possibility of impaired wound healing or dehiscence while being treated with AFINITOR [see Warnings and Precautions (5.5)].
- Laboratory Tests and Monitoring
- Inform patients of the need to monitor blood chemistry and hematology prior to the start of AFINITOR therapy and periodically thereafter [see Warnings and Precautions (5.7)].
- Drug-drug Interactions
- Advise patients to inform their healthcare providers of all concomitant medications, including over-the-counter medications and dietary supplements. Inform the patients to avoid concomitant administration of strong CYP3A4/PgP inhibitors or inducers while on AFINITOR treatment [see Dosage and Administration (2.2, 2.5), Warnings and Precautions (5.8), and Drug Interactions (7.1, 7.2)].
- Vaccinations
- Advise patients to avoid the use of live vaccines and close contact with those who have received live vaccines [see Warnings and Precautions (5.10)].
- Embryo-fetal Toxicity
- Advise female patients of childbearing potential that AFINITOR may cause fetal harm and that a highly effective method of contraception should be used during therapy with AFINITOR and for up to 8 weeks after ending treatment [see Warnings and Precautions (5.11)].
- Safe Handling Practices for AFINITOR DISPERZ
- Advise patients and their caregivers to read and carefully follow the FDA approved AFINITOR DISPERZ “Instructions for Use”.
- Dosing Instructions
- Inform patients to take AFINITOR Tablets orally once daily at the same time every day, either consistently with food or consistently without food. :*Inform patients that AFINITOR Tablets should be swallowed whole with a glass of water.
- Inform patients to take AFINITOR DISPERZ orally once daily at the same time every day as a suspension. Refer patients to the “Instructions for Use” pamphlet for additional information regarding these procedures.
- Instruct patients that if they miss a dose of AFINITOR, they may still take it up to 6 hours after the time they would normally take it. If more than 6 hours have elapsed, they should be instructed to skip the dose for that day. The next day, they should take AFINITOR at the usual time. Warn patients to not take 2 doses to make up for the one that they missed.
Precautions with Alcohol
- Alcohol-Everolimus interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Afinitor®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ R.N Formica Jra, K.M Lorberb, A.L Friedmanb, M.J Biaa, F Lakkisa, J.D Smitha, M.I Lorber (March 2004). "The evolving experience using everolimus in clinical transplantation". Elsevier. 36 (2): S495–S499. doi:10.1016/j.transproceed.2004.01.015.
- ↑ "AFINITOR (everolimus) tablet AFINITOR DISPERZ (everolimus) tablet, for suspension [Novartis Pharmaceuticals Corporation]".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Everolimus |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus19.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus20.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus21.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus22.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus23.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus24.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus25.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus26.png
}}
{{#subobject:
|Label Page=Everolimus |Label Name=Everolimus27.png
}} [[Category:]]
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 errors: external links
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug