Etonogestrel: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{GP}} | |authorTag={{GP}} | ||
|genericName=Etonogestrel | |||
|aOrAn=a | |||
|drugClass=progestin | |||
|indicationType=prophylaxis | |||
|indication=pregnancy | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=Etonogestrel is indicated for use by women to prevent pregnancy. | |||
* Dosage: | |||
:* Etonogestrel is insert subdermally just under the skin at the inner side of the non-dominant upper arm. | |||
:* Etonogestrel must be removed no later than by the end of the third year. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Etonogestrel in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Etonogestrel in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Etonogestrel in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Etonogestrel in adult patients. | ||
|fdaLIADPed=* Clinical studies have not been conducted in women less than 18 years of age | |||
* Safety and efficacy are expected to be identical for postpubertal adolescents | |||
* Use before menarche is not indicated | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Etonogestrel in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Etonogestrel in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Etonogestrel in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Etonogestrel in pediatric patients. | ||
|contraindications=Etonogestrel should not be used in women who have | |||
* Known or suspected pregnancy | |||
* Current or past history of thrombosis or thromboembolic disorders | |||
* Liver tumors, benign or malignant, or active liver disease | |||
* Undiagnosed abnormal genital bleeding | |||
* Known or suspected breast cancer, personal history of breast cancer, or other progestin-sensitive cancer, now or in the past | |||
* Allergic reaction to any of the components of IMPLANON | |||
|warnings=Complications of Insertion and Removal | |||
IMPLANON should be inserted subdermally so that it will be palpable after insertion, and this should be confirmed by palpation immediately after insertion. Failure to insert IMPLANON properly may go unnoticed unless it is palpated immediately after insertion. Undetected failure to insert the implant may lead to an unintended pregnancy. Complications related to insertion and removal procedures, such as pain, paresthesias, bleeding, hematoma, scarring or infection, may occur. Occasionally in post-marketing use, implant insertions have failed because the implant fell out of the needle or remained in the needle during insertion. | |||
If IMPLANON is inserted too deeply (intramuscular or in the fascia), neural or vascular injury may occur. To reduce the risk of neural or vascular injury, IMPLANON should be inserted at the inner side of the non-dominant upper arm about 8-10 cm (3-4 inches) above the medial epicondyle of the humerus. IMPLANON should be inserted subdermally just under the skin to avoid the large blood vessels and nerves that lie deeper in the subcutaneous tissues in the sulcus between the triceps and biceps muscles. Deep insertions of IMPLANON have been associated with paraesthesia (due to neural injury) and migration of the implant (due to intramuscular or fascial insertion), and in a very few cases with intravascular insertion. If infection develops at the insertion site, start suitable treatment. If the infection persists, the implant should be removed. Incomplete insertions or infections may lead to expulsion. In postmarketing use there have been cases of failure to localize and remove the implant, probably due to deep insertion. There has been 1 case of an intravascular insertion reported post-marketing which led to inability to remove the implant. | |||
Implant removal may be difficult or impossible if the implant is not inserted correctly, is inserted too deeply, not palpable, encased in fibrous tissue, or has migrated. Deep insertions may lead to difficult localization of the implant and may also result in the need for a surgical procedure in an operating room in order to remove the implant. Exploratory surgery without knowledge of the exact location of the implant is strongly discouraged. Removal of deeply inserted implants should be conducted with caution in order to prevent injury to deeper neural or vascular structures in the arm and be performed by healthcare providers familiar with the anatomy of the arm. Failure to remove the implant may result in continued effects of etonogestrel, such as compromised fertility, ectopic pregnancy, or persistence or occurrence of a drug-related adverse event. | |||
5.2 Changes in Menstrual Bleeding Patterns | |||
After starting IMPLANON, women are likely to have a change from their normal menstrual bleeding pattern. These may include changes in bleeding frequency (absent, less, more frequent or continuous), intensity (reduced or increased) or duration. In clinical trials, bleeding patterns ranged from amenorrhea (1 in 5 women) to frequent and/or prolonged bleeding (1 in 5 women). The bleeding pattern experienced during the first three months of IMPLANON use is broadly predictive of the future bleeding pattern for many women. Women should be counseled regarding the bleeding pattern changes they may experience so that they know what to expect. Abnormal bleeding should be evaluated as needed to exclude pathologic conditions or pregnancy. | |||
In clinical studies of IMPLANON, reports of changes in bleeding pattern were the most common reason for stopping treatment (11.1%). Irregular bleeding (10.8%) was the single most common reason women stopped treatment, while amenorrhea (0.3%) was cited less frequently. In these studies, women had an average of 17.7 days of bleeding or spotting every 90 days (based on 3,315 intervals of 90 days recorded by 780 patients). The percentages of patients having 0, 1-7, 8-21, or >21 days of spotting or bleeding over a 90-day interval while using the IMPLANON implant are shown in TABLE 1. | |||
[[etonogestrel_tabla 1]] | |||
Bleeding patterns observed with use of IMPLANON for up to 2 years, and the proportion of 90-day intervals with these bleeding patterns, are summarized in TABLE 2. | |||
[[Etonogestrel tbala 2]] | |||
Ectopic Pregnancies | |||
As with all progestin-only contraceptive products, be alert to the possibility of an ectopic pregnancy among women using IMPLANON who become pregnant or complain of lower abdominal pain. Although ectopic pregnancies are uncommon among women using IMPLANON, a pregnancy that occurs in a woman using IMPLANON may be more likely to be ectopic than a pregnancy occurring in a woman using no contraception. | |||
5.4 Thrombotic and Other Vascular Events | |||
The use of combination hormonal contraceptives (progestin plus estrogen) increases the risk of vascular events, including arterial events (strokes and myocardial infarctions) or deep venous thrombotic events (venous thromboembolism, deep venous thrombosis, retinal vein thrombosis, and pulmonary embolism). IMPLANON is a progestin-only contraceptive. It is unknown whether this increased risk is applicable to etonogestrel alone. It is recommended, however, that women with risk factors known to increase the risk of venous and arterial thromboembolism be carefully assessed. | |||
There have been postmarketing reports of serious arterial and venous thromboembolic events, including cases of pulmonary emboli (some fatal), deep vein thrombosis, myocardial infarction, and strokes, in women using IMPLANON. IMPLANON should be removed in the event of a thrombosis. | |||
Due to the risk of thromboembolism associated with pregnancy and immediately following delivery, IMPLANON should not be used prior to 21 days postpartum. Women with a history of thromboembolic disorders should be made aware of the possibility of a recurrence. | |||
Evaluate for retinal vein thrombosis immediately if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. | |||
Consider removal of the IMPLANON implant in case of long-term immobilization due to surgery or illness. | |||
5.5 Ovarian Cysts | |||
If follicular development occurs, atresia of the follicle is sometimes delayed, and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally, these enlarged follicles disappear spontaneously. On rare occasion, surgery may be required. | |||
5.6 Carcinoma of the Breast and Reproductive Organs | |||
Women who currently have or have had breast cancer should not use hormonal contraception because breast cancer may be hormonally sensitive [see CONTRAINDICATIONS (4)]. Some studies suggest that the use of combination hormonal contraceptives might increase the incidence of breast cancer; however, other studies have not confirmed such findings. | |||
Some studies suggest that the use of combination hormonal contraceptives is associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which such findings are due to differences in sexual behavior and other factors. | |||
Women with a family history of breast cancer or who develop breast nodules should be carefully monitored. | |||
5.7 Liver Disease | |||
Disturbances of liver function may necessitate the discontinuation of hormonal contraceptive use until markers of liver function return to normal. Remove IMPLANON if jaundice develops. | |||
Hepatic adenomas are associated with combination hormonal contraceptives use. An estimate of the attributable risk is 3.3 cases per 100,000 for combination hormonal contraceptives users. It is not known whether a similar risk exists with progestin-only methods like IMPLANON. | |||
The progestin in IMPLANON may be poorly metabolized in women with liver impairment. Use of IMPLANON in women with active liver disease or liver cancer is contraindicated [see CONTRAINDICATIONS (4)]. | |||
5.8 Weight Gain | |||
In clinical studies, mean weight gain in US IMPLANON users was 2.8 pounds after 1 year and 3.7 pounds after 2 years. How much of the weight gain was related to the implant is unknown. In studies, 2.3% of the users reported weight gain as the reason for having the implant removed. | |||
5.9 Elevated Blood Pressure | |||
Women with a history of hypertension-related diseases or renal disease should be discouraged from using hormonal contraception. For women with well-controlled hypertension, use of IMPLANON can be considered. Women with hypertension using IMPLANON should be closely monitored. lf sustained hypertension develops during the use of IMPLANON, or if a significant increase in blood pressure does not respond adequately to antihypertensive therapy, IMPLANON should be removed. | |||
5.10 Gallbladder Disease | |||
Studies suggest a small increased relative risk of developing gallbladder disease among combination hormonal contraceptive users. It is not known whether a similar risk exists with progestin-only methods like IMPLANON. | |||
5.11 Carbohydrate and Lipid Metabolic Effects | |||
Use of IMPLANON may induce mild insulin resistance and small changes in glucose concentrations of unknown clinical significance. Carefully monitor prediabetic and diabetic women using IMPLANON. | |||
Women who are being treated for hyperlipidemia should be followed closely if they elect to use IMPLANON. Some progestins may elevate LDL levels and may render the control of hyperlipidemia more difficult. | |||
5.12 Depressed Mood | |||
Women with a history of depressed mood should be carefully observed. Consideration should be given to removing IMPLANON in patients who become significantly depressed. | |||
5.13 Return to Ovulation | |||
In clinical trials with IMPLANON, the etonogestrel levels in blood decreased below sensitivity of the assay by one week after removal of the implant. In addition, pregnancies were observed to occur as early as 7 to 14 days after removal. Therefore, a woman should re-start contraception immediately after removal of the implant if continued contraceptive protection is desired. | |||
5.14 Fluid Retention | |||
Hormonal contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention. It is unknown if IMPLANON causes fluid retention. | |||
5.15 Contact Lenses | |||
Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist. | |||
|alcohol=Alcohol-Etonogestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Etonogestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 15:38, 2 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Etonogestrel is a progestin that is FDA approved for the prophylaxis of pregnancy. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Etonogestrel is indicated for use by women to prevent pregnancy.
- Dosage:
- Etonogestrel is insert subdermally just under the skin at the inner side of the non-dominant upper arm.
- Etonogestrel must be removed no later than by the end of the third year.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Etonogestrel in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Etonogestrel in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Clinical studies have not been conducted in women less than 18 years of age
- Safety and efficacy are expected to be identical for postpubertal adolescents
- Use before menarche is not indicated
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Etonogestrel in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Etonogestrel in pediatric patients.
Contraindications
Etonogestrel should not be used in women who have
- Known or suspected pregnancy
- Current or past history of thrombosis or thromboembolic disorders
- Liver tumors, benign or malignant, or active liver disease
- Undiagnosed abnormal genital bleeding
- Known or suspected breast cancer, personal history of breast cancer, or other progestin-sensitive cancer, now or in the past
- Allergic reaction to any of the components of IMPLANON
Warnings
Complications of Insertion and Removal IMPLANON should be inserted subdermally so that it will be palpable after insertion, and this should be confirmed by palpation immediately after insertion. Failure to insert IMPLANON properly may go unnoticed unless it is palpated immediately after insertion. Undetected failure to insert the implant may lead to an unintended pregnancy. Complications related to insertion and removal procedures, such as pain, paresthesias, bleeding, hematoma, scarring or infection, may occur. Occasionally in post-marketing use, implant insertions have failed because the implant fell out of the needle or remained in the needle during insertion.
If IMPLANON is inserted too deeply (intramuscular or in the fascia), neural or vascular injury may occur. To reduce the risk of neural or vascular injury, IMPLANON should be inserted at the inner side of the non-dominant upper arm about 8-10 cm (3-4 inches) above the medial epicondyle of the humerus. IMPLANON should be inserted subdermally just under the skin to avoid the large blood vessels and nerves that lie deeper in the subcutaneous tissues in the sulcus between the triceps and biceps muscles. Deep insertions of IMPLANON have been associated with paraesthesia (due to neural injury) and migration of the implant (due to intramuscular or fascial insertion), and in a very few cases with intravascular insertion. If infection develops at the insertion site, start suitable treatment. If the infection persists, the implant should be removed. Incomplete insertions or infections may lead to expulsion. In postmarketing use there have been cases of failure to localize and remove the implant, probably due to deep insertion. There has been 1 case of an intravascular insertion reported post-marketing which led to inability to remove the implant.
Implant removal may be difficult or impossible if the implant is not inserted correctly, is inserted too deeply, not palpable, encased in fibrous tissue, or has migrated. Deep insertions may lead to difficult localization of the implant and may also result in the need for a surgical procedure in an operating room in order to remove the implant. Exploratory surgery without knowledge of the exact location of the implant is strongly discouraged. Removal of deeply inserted implants should be conducted with caution in order to prevent injury to deeper neural or vascular structures in the arm and be performed by healthcare providers familiar with the anatomy of the arm. Failure to remove the implant may result in continued effects of etonogestrel, such as compromised fertility, ectopic pregnancy, or persistence or occurrence of a drug-related adverse event.
5.2 Changes in Menstrual Bleeding Patterns After starting IMPLANON, women are likely to have a change from their normal menstrual bleeding pattern. These may include changes in bleeding frequency (absent, less, more frequent or continuous), intensity (reduced or increased) or duration. In clinical trials, bleeding patterns ranged from amenorrhea (1 in 5 women) to frequent and/or prolonged bleeding (1 in 5 women). The bleeding pattern experienced during the first three months of IMPLANON use is broadly predictive of the future bleeding pattern for many women. Women should be counseled regarding the bleeding pattern changes they may experience so that they know what to expect. Abnormal bleeding should be evaluated as needed to exclude pathologic conditions or pregnancy.
In clinical studies of IMPLANON, reports of changes in bleeding pattern were the most common reason for stopping treatment (11.1%). Irregular bleeding (10.8%) was the single most common reason women stopped treatment, while amenorrhea (0.3%) was cited less frequently. In these studies, women had an average of 17.7 days of bleeding or spotting every 90 days (based on 3,315 intervals of 90 days recorded by 780 patients). The percentages of patients having 0, 1-7, 8-21, or >21 days of spotting or bleeding over a 90-day interval while using the IMPLANON implant are shown in TABLE 1.
Bleeding patterns observed with use of IMPLANON for up to 2 years, and the proportion of 90-day intervals with these bleeding patterns, are summarized in TABLE 2.
Ectopic Pregnancies As with all progestin-only contraceptive products, be alert to the possibility of an ectopic pregnancy among women using IMPLANON who become pregnant or complain of lower abdominal pain. Although ectopic pregnancies are uncommon among women using IMPLANON, a pregnancy that occurs in a woman using IMPLANON may be more likely to be ectopic than a pregnancy occurring in a woman using no contraception.
5.4 Thrombotic and Other Vascular Events The use of combination hormonal contraceptives (progestin plus estrogen) increases the risk of vascular events, including arterial events (strokes and myocardial infarctions) or deep venous thrombotic events (venous thromboembolism, deep venous thrombosis, retinal vein thrombosis, and pulmonary embolism). IMPLANON is a progestin-only contraceptive. It is unknown whether this increased risk is applicable to etonogestrel alone. It is recommended, however, that women with risk factors known to increase the risk of venous and arterial thromboembolism be carefully assessed.
There have been postmarketing reports of serious arterial and venous thromboembolic events, including cases of pulmonary emboli (some fatal), deep vein thrombosis, myocardial infarction, and strokes, in women using IMPLANON. IMPLANON should be removed in the event of a thrombosis.
Due to the risk of thromboembolism associated with pregnancy and immediately following delivery, IMPLANON should not be used prior to 21 days postpartum. Women with a history of thromboembolic disorders should be made aware of the possibility of a recurrence.
Evaluate for retinal vein thrombosis immediately if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions.
Consider removal of the IMPLANON implant in case of long-term immobilization due to surgery or illness.
5.5 Ovarian Cysts If follicular development occurs, atresia of the follicle is sometimes delayed, and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally, these enlarged follicles disappear spontaneously. On rare occasion, surgery may be required.
5.6 Carcinoma of the Breast and Reproductive Organs Women who currently have or have had breast cancer should not use hormonal contraception because breast cancer may be hormonally sensitive [see CONTRAINDICATIONS (4)]. Some studies suggest that the use of combination hormonal contraceptives might increase the incidence of breast cancer; however, other studies have not confirmed such findings.
Some studies suggest that the use of combination hormonal contraceptives is associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which such findings are due to differences in sexual behavior and other factors.
Women with a family history of breast cancer or who develop breast nodules should be carefully monitored.
5.7 Liver Disease Disturbances of liver function may necessitate the discontinuation of hormonal contraceptive use until markers of liver function return to normal. Remove IMPLANON if jaundice develops.
Hepatic adenomas are associated with combination hormonal contraceptives use. An estimate of the attributable risk is 3.3 cases per 100,000 for combination hormonal contraceptives users. It is not known whether a similar risk exists with progestin-only methods like IMPLANON.
The progestin in IMPLANON may be poorly metabolized in women with liver impairment. Use of IMPLANON in women with active liver disease or liver cancer is contraindicated [see CONTRAINDICATIONS (4)].
5.8 Weight Gain In clinical studies, mean weight gain in US IMPLANON users was 2.8 pounds after 1 year and 3.7 pounds after 2 years. How much of the weight gain was related to the implant is unknown. In studies, 2.3% of the users reported weight gain as the reason for having the implant removed.
5.9 Elevated Blood Pressure Women with a history of hypertension-related diseases or renal disease should be discouraged from using hormonal contraception. For women with well-controlled hypertension, use of IMPLANON can be considered. Women with hypertension using IMPLANON should be closely monitored. lf sustained hypertension develops during the use of IMPLANON, or if a significant increase in blood pressure does not respond adequately to antihypertensive therapy, IMPLANON should be removed.
5.10 Gallbladder Disease Studies suggest a small increased relative risk of developing gallbladder disease among combination hormonal contraceptive users. It is not known whether a similar risk exists with progestin-only methods like IMPLANON.
5.11 Carbohydrate and Lipid Metabolic Effects Use of IMPLANON may induce mild insulin resistance and small changes in glucose concentrations of unknown clinical significance. Carefully monitor prediabetic and diabetic women using IMPLANON.
Women who are being treated for hyperlipidemia should be followed closely if they elect to use IMPLANON. Some progestins may elevate LDL levels and may render the control of hyperlipidemia more difficult.
5.12 Depressed Mood Women with a history of depressed mood should be carefully observed. Consideration should be given to removing IMPLANON in patients who become significantly depressed.
5.13 Return to Ovulation In clinical trials with IMPLANON, the etonogestrel levels in blood decreased below sensitivity of the assay by one week after removal of the implant. In addition, pregnancies were observed to occur as early as 7 to 14 days after removal. Therefore, a woman should re-start contraception immediately after removal of the implant if continued contraceptive protection is desired.
5.14 Fluid Retention Hormonal contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention. It is unknown if IMPLANON causes fluid retention.
5.15 Contact Lenses Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Etonogestrel Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Etonogestrel Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Etonogestrel Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Etonogestrel in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Etonogestrel in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Etonogestrel during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Etonogestrel in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Etonogestrel in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Etonogestrel in geriatric settings.
Gender
There is no FDA guidance on the use of Etonogestrel with respect to specific gender populations.
Race
There is no FDA guidance on the use of Etonogestrel with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Etonogestrel in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Etonogestrel in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Etonogestrel in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Etonogestrel in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Etonogestrel Administration in the drug label.
Monitoring
There is limited information regarding Etonogestrel Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Etonogestrel and IV administrations.
Overdosage
There is limited information regarding Etonogestrel overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Etonogestrel Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Etonogestrel Mechanism of Action in the drug label.
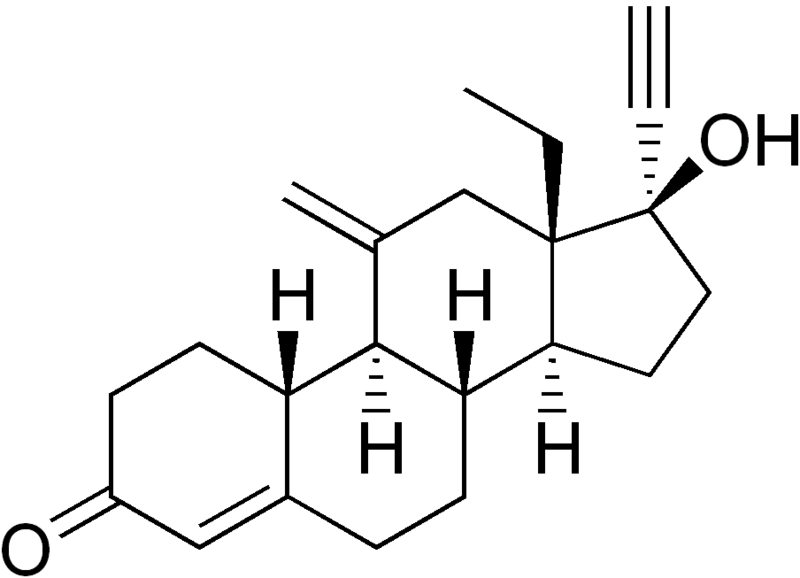
Structure
There is limited information regarding Etonogestrel Structure in the drug label.
Pharmacodynamics
There is limited information regarding Etonogestrel Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Etonogestrel Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Etonogestrel Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Etonogestrel Clinical Studies in the drug label.
How Supplied
There is limited information regarding Etonogestrel How Supplied in the drug label.
Storage
There is limited information regarding Etonogestrel Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Etonogestrel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Etonogestrel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Etonogestrel Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Etonogestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Etonogestrel Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Etonogestrel Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Subdermal as slow-release implant |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (P450 3A4) |
| Elimination half-life | 25 hours |
| Excretion | Urinary (majority) and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C22H28O2 |
| Molar mass | 324.457 g/mol |
|
WikiDoc Resources for Etonogestrel |
|
Articles |
|---|
|
Most recent articles on Etonogestrel Most cited articles on Etonogestrel |
|
Media |
|
Powerpoint slides on Etonogestrel |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Etonogestrel at Clinical Trials.gov Clinical Trials on Etonogestrel at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Etonogestrel
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Etonogestrel Discussion groups on Etonogestrel Patient Handouts on Etonogestrel Directions to Hospitals Treating Etonogestrel Risk calculators and risk factors for Etonogestrel
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Etonogestrel |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Etonogestrel is a molecule used in hormonal contraceptives, most notably the subdermal implant Implanon.
Etonogestrel, the specific progestin used in NuvaRing, is the active metabolite of the inactive prodrug desogestrel, one of two third-generation progestins found in some epidemiological studies of combined oral contraceptive pills to be associated with a higher risk of venous thrombosis than combined oral contraceptive pills containing certain second-generation progestins. Because hormones are released continuously from NuvaRing, peak and total estrogen and progestin doses are significantly lower than with combined oral contraceptives, although it is not known whether this lowers the risk of blood clots.
See also
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Progestagens
- Endocrinology