Estramustine: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{AV}} | ||
|genericName=Estramustine | |||
|aOrAn=an | |||
|drugClass=antineoplastic agent | |||
|indicationType=treatment | |||
|genericName= | |indication=patients with [[prostate cancer|metastatic and/or progressive carcinoma of the prostate]] | ||
|adverseReactions=[[edema]], [[tenderness|breast tenderness]], [[diarrhea]], [[gastritis|gastrointestinal irritation]], [[nausea]], [[cramp|leg cramp]] and [[dyspnea]] | |||
|aOrAn= | |||
|drugClass= | |||
|indication= | |||
| | |||
|adverseReactions= | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle=Title | |||
|blackBoxWarningTitle= | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | * Content | ||
| Line 42: | Line 16: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Carcinoma of the prostate===== | ||
Estramustine Capsules are indicated in the palliative treatment of patients with [[prostate cancer|metastatic and/or progressive carcinoma of the prostate]]. | |||
=====Dosing Information===== | |||
*The recommended daily dose is 14 mg per kg of body weight (ie, one 140 mg capsule for each 10 kg or 22 lb of body weight), given in 3 or 4 divided doses. Most patients in studies in the United States have been treated at a dosage range of 10 to 16 mg per kg per day. | |||
*Patients should be instructed to take Estramustine Capsules at least 1 hour before or 2 hours after meals. Estramustine should be swallowed with water. Milk, milk products, and calcium-rich foods or drugs (such as [[antacids|calcium-containing antacids]]) must not be taken simultaneously with Estramustine . | |||
* | *Patients should be treated for 30 to 90 days before the physician determines the possible benefits of continued therapy. Therapy should be continued as long as the favorable response lasts. Some patients have been maintained on therapy for more than 3 years at doses ranging from 10 to 16 mg per kg of body weight per day. | ||
*Procedures for proper handling and disposal of [[anticancer drugs]] should be considered. Several guidelines on this subject have been published.1–8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Estramustine in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Estramustine in adult patients. | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Estramustine in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Estramustine in pediatric patients. | |||
|contraindications=* Estramustine Capsules should not be used in patients with any of the following conditions: | |||
:*Known [[hypersensitivity]] to either [[estradiol]] or to [[nitrogen mustard]]. | |||
:*Active [[thrombophlebitis]] or [[thromboembolic disorders]], except in those cases where the actual tumor mass is the cause of the [[thromboembolism |thromboembolic phenomenon]] and the physician feels the benefits of therapy may outweigh the risks. | |||
<!--Warnings--> | |||
|warnings=*It has been shown that there is an increased risk of [[thrombosis]], including fatal and nonfatal [[myocardial infarction]], in men receiving [[estrogens]] for [[prostatic cancer]]. Estramustine Capsules should be used with caution in patients with a history of [[thrombophlebitis]], [[thrombosis]], or [[thromboembolic disorders]], especially if they were associated with [[estrogen|estrogen therapy]]. Caution should also be used in patients with cerebral vascular or [[coronary artery disease]]. | |||
*[[Glucose Tolerance]]—Because [[glucose tolerance]] may be decreased, diabetic patients should be carefully observed while receiving Estramustine . | |||
*Elevated Blood Pressure—Because [[hypertension]] may occur, [[blood pressure]] should be monitored periodically. | |||
====Precautions==== | |||
===== | |||
=====General===== | |||
* | *Fluid Retention. Exacerbation of preexisting or incipient peripheral edema or congestive heart disease has been seen in some patients receiving therapy with Estramustine Capsules. Other conditions which might be influenced by [[fluid retention]], such as [[epilepsy]], [[migraine]], or renal dysfunction, require careful observation. | ||
* | *Estramustine may be poorly metabolized in patients with impaired liver function and should be administered with caution in such patients. | ||
*Because Estramustine may influence the metabolism of [[calcium]] and [[phosphorus]], it should be used with caution in patients with metabolic bone diseases that are associated with [[hypercalcemia]] or in patients with [[renal insufficiency]]. Patients with [[prostate cancer]] and [[osteoblastic metastases]] are at risk for [[hypocalcemia]] and should have [[calcium|calcium levels]] closely monitored. | |||
*[[Gynecomastia]] and impotence are known estrogenic effects. | |||
*Allergic reactions and [[angioedema]] at times involving the airway have been reported. | |||
=====Information for the Patient===== | |||
| | *Because of the possibility of mutagenic effects, patients should be advised to use [[contraception|contraceptive measures]]. | ||
===== | =====Laboratory Tests===== | ||
* | *Certain endocrine and liver function tests may be affected by [[estrogen|estrogen-containing drugs]]. Estramustine may depress [[testosterone]] levels. Abnormalities of [[hepatic enzymes]] and of [[bilirubin]] have occurred in patients receiving Estramustine . Such tests should be done at appropriate intervals during therapy and repeated after the drug has been withdrawn for two months. | ||
=====Food/Drug Interaction===== | |||
===== | |||
*Milk, milk products, and [[calcium-rich foods]] or drugs may impair the absorption of Estramustine . | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=*In a randomized, double-blind trial comparing therapy with Estramustine Capsules in 93 patients (11.5 to 15.9 mg/kg/day) or [[diethylstilbestrol]] (DES) in 93 patients (3.0 mg/day), the following adverse effects were reported: | |||
| | [[File:Estramustine02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Estramustine in the drug label. | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=<!--Use in Specific Populations--> | |||
|drugInteractions= | |useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Estramustine in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of Estramustine during labor and delivery. | |||
|useInNursing=There is no FDA guidance on the use of Estramustine with respect to nursing mothers. | |||
|useInPed=There is no FDA guidance on the use of Estramustine with respect to pediatric patients. | |||
|useInGeri=There is no FDA guidance on the use of Estramustine with respect to geriatric patients. | |||
<!--Use in Specific Populations--> | |useInGender=There is no FDA guidance on the use of Estramustine with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of Estramustine with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of Estramustine in patients with [[renal impairment]]. | |||
|useInHepaticImpair=There is no FDA guidance on the use of Estramustine in patients with [[hepatic impairment]]. | |||
|useInReproPotential=There is no FDA guidance on the use of Estramustine in women of reproductive potentials and males. | |||
|useInPregnancyAUS= | |useInImmunocomp=There is no FDA guidance one the use of Estramustine in patients who are [[immunocompromised]]. | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of | |||
|useInNursing= | |||
There is no FDA guidance on the use of | |||
|useInPed= | |||
There is no FDA guidance on the use of | |||
|useInGeri= | |||
There is no FDA guidance on the use of | |||
|useInGender= | |||
There is no FDA guidance on the use of | |||
|useInRace= | |||
There is no FDA guidance on the use of | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |||
|administration= | |monitoring=There is limited information regarding <i>Monitoring</i> of Estramustine in the drug label. | ||
* Oral | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of Estramustine in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose=*Although there has been no experience with overdosage to date, it is reasonable to expect that such episodes may produce pronounced manifestations of the known adverse reactions. In the event of overdosage, the gastric contents should be evacuated by [[gastric lavage]] and symptomatic therapy should be initiated. Hematologic and hepatic parameters should be monitored for at least 6 weeks after overdosage of Estramustine Capsules | |||
|overdose= | |||
* | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 461095578 | |||
| IUPAC_name = (17β)-17-Hydroxyestra-1(10),2,4-trien-3-yl bis(2-chloroethyl)carbamate | |||
| image = Estramustine00.png | |||
| width = 250px | |||
| image2 = Estramustine000.gif | |||
| width2 = 200px | |||
<!--Clinical data--> | |||
| tradename = Estramustine | |||
| Drugs.com = {{drugs.com|monograph|Estramustine }} | |||
| MedlinePlus = a608046 | |||
| licence_US = Estramustine | |||
| pregnancy_AU = D | |||
| pregnancy_US = X | |||
| legal_AU = S4 | |||
| legal_CA = Rx-only | |||
| legal_US = Rx-only | |||
| legal_UK = POM | |||
| routes_of_administration = | |||
| | <!--Pharmacokinetic data--> | ||
| bioavailability = 75%<ref name = MSR>{{cite web|title=Estramustine (estramustine) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=8 February 2014|url=http://reference.medscape.com/drug/Estramustine -estramustine-342085#showall}}</ref> | |||
| protein_bound = | |||
| metabolism = [[Hepatic]] to estradiol and estrone<ref name = MSR/> | |||
| elimination_half-life = 15-24 hours<ref name = MSR/> | |||
| excretion = Faeces (2.9-4.8%)<ref name = MSR/> | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 2998-57-4 | |||
| ATC_prefix = L01 | |||
| ATC_suffix = XX11 | |||
| PubChem = 259331 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB01196 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 227635 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 35LT29625A | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D04066 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 4868 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1575 | |||
<!--Chemical data--> | |||
| C = 23 | H = 31 | Cl = 2 | N = 1 | O = 3 | |||
| molecular_weight = 440.403 [[Gram|g]]/[[Mole (unit)|mol]] | |||
| smiles = ClCCN(C(=O)Oc1cc3c(cc1)[C@H]2CC[C@@]4([C@@H](O)CC[C@H]4[C@@H]2CC3)C)CCCl | |||
| InChI = 1/C23H31Cl2NO3/c1-23-9-8-18-17-5-3-16(29-22(28)26(12-10-24)13-11-25)14-15(17)2-4-19(18)20(23)6-7-21(23)27/h3,5,14,18-21,27H,2,4,6-13H2,1H3/t18-,19-,20+,21+,23+/m1/s1 | |||
| InChIKey = FRPJXPJMRWBBIH-RBRWEJTLBB | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C23H31Cl2NO3/c1-23-9-8-18-17-5-3-16(29-22(28)26(12-10-24)13-11-25)14-15(17)2-4-19(18)20(23)6-7-21(23)27/h3,5,14,18-21,27H,2,4,6-13H2,1H3/t18-,19-,20+,21+,23+/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = FRPJXPJMRWBBIH-RBRWEJTLSA-N | |||
}} | |||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=* Prolonged treatment with estramustine phosphate produces elevated total plasma concentrations of [[estradiol]] that fall within ranges similar to the [[elevated estradiol]] levels found in [[prostatic cancer]] patients given conventional [[estradiol]] therapy. | |||
|mechAction= | |||
* | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* Estramustine phosphate sodium, an [[chemotherapy|antineoplastic agent]], is an off-white powder readily soluble in water. Estramustine Capsules are white and opaque, each containing estramustine phosphate sodium as the disodium salt monohydrate equivalent to 140 mg estramustine phosphate, for oral administration. Each capsule also contains [[magnesium stearate]], [[silicon dioxide]], sodium lauryl sulfate, and [[talc]]. Gelatin capsule shells contain the following pigment: [[titanium dioxide]]. | |||
*Chemically, estramustine phosphate sodium is estra-1,3,5(10)-triene-3,17-diol(17β)-,3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. It is also referred to as estradiol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. Estramustine phosphate sodium has an empiric formula of C23H30Cl2NNa2O6P•H2O, a calculated molecular weight of 582.4, and the following structural formula: | |||
: [[File:Estramustine01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
: [[File: | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of Estramustine in the drug label. | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=*Estramustine phosphate (Figure 1) is a molecule combining [[estradiol]] and [[nitrogen mustard|nornitrogen mustard]] by a carbamate link. The molecule is phosphorylated to make it water soluble.There is limited information regarding <i>Pharmacokinetics</i> of Estramustine in the drug label. | |||
*Estramustine phosphate taken orally is readily dephosphorylated during absorption, and the major metabolites in plasma are estramustine (Figure 2), the [[estrone]] analog (Figure 3), [[estradiol]], and [[estrone]]. | |||
*Estrogenic effects, as demonstrated by changes in circulating levels of steroids and pituitary hormones, are similar in patients treated with either estramustine phosphate or [[estradiol|conventional estradiol]]. | |||
*The metabolic urinary patterns of the [[estradiol]] moiety of estramustine phosphate and [[estradiol]] itself are very similar, although the metabolites derived from estramustine phosphate are excreted at a slower rate. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic= | |nonClinToxic= | ||
=====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
*Long-term continuous administration of [[estrogens]] in certain animal species increases the frequency of carcinomas of the breast and liver. Compounds structurally similar to Estramustine are carcinogenic in mice. Carcinogenic studies of Estramustine have not been conducted in man. Although testing by the [[Ames method]] failed to demonstrate mutagenicity for estramustine phosphate sodium, it is known that both [[estradiol]] and [[nitrogen mustard]] are mutagenic. For this reason and because some patients who had been impotent while on [[estrogen|estrogen therapy]] have regained potency while taking Estramustine , the patient should be advised to use contraceptive measures. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of Estramustine in the drug label. | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* White opaque capsules, each containing estramustine phosphate sodium as the disodium salt monohydrate equivalent to 140 mg estramustine phosphate—bottle of 100 (NDC 0013-0132-02). | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|storage='''''NOTE''''' | |||
|fdaPatientInfo= | *Estramustine Capsules should be stored in the refrigerator at 36° to 46°F (2° to 8°C). | ||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of Estramustine in the drug label. | |||
There is limited information regarding <i>Patient Counseling Information</i> of | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-Estramustine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol- | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=*Emcyt® | |||
|brandNames= | |||
* | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage | {{PillImage | ||
|fileName=No image.jpg | |fileName=No image.jpg | ||
}} | |||
{{LabelImage | |||
| | |fileName=Estramustine03.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=Estramustine04.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=Estramustine05.png | ||
}} | }} | ||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Chemotherapeutic agents]] | |||
Latest revision as of 12:46, 8 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Estramustine is an antineoplastic agent that is FDA approved for the treatment of patients with metastatic and/or progressive carcinoma of the prostate. Common adverse reactions include edema, breast tenderness, diarrhea, gastrointestinal irritation, nausea, leg cramp and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Carcinoma of the prostate
Estramustine Capsules are indicated in the palliative treatment of patients with metastatic and/or progressive carcinoma of the prostate.
Dosing Information
- The recommended daily dose is 14 mg per kg of body weight (ie, one 140 mg capsule for each 10 kg or 22 lb of body weight), given in 3 or 4 divided doses. Most patients in studies in the United States have been treated at a dosage range of 10 to 16 mg per kg per day.
- Patients should be instructed to take Estramustine Capsules at least 1 hour before or 2 hours after meals. Estramustine should be swallowed with water. Milk, milk products, and calcium-rich foods or drugs (such as calcium-containing antacids) must not be taken simultaneously with Estramustine .
- Patients should be treated for 30 to 90 days before the physician determines the possible benefits of continued therapy. Therapy should be continued as long as the favorable response lasts. Some patients have been maintained on therapy for more than 3 years at doses ranging from 10 to 16 mg per kg of body weight per day.
- Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1–8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Estramustine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Estramustine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Estramustine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Estramustine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Estramustine in pediatric patients.
Contraindications
- Estramustine Capsules should not be used in patients with any of the following conditions:
- Known hypersensitivity to either estradiol or to nitrogen mustard.
- Active thrombophlebitis or thromboembolic disorders, except in those cases where the actual tumor mass is the cause of the thromboembolic phenomenon and the physician feels the benefits of therapy may outweigh the risks.
Warnings
- It has been shown that there is an increased risk of thrombosis, including fatal and nonfatal myocardial infarction, in men receiving estrogens for prostatic cancer. Estramustine Capsules should be used with caution in patients with a history of thrombophlebitis, thrombosis, or thromboembolic disorders, especially if they were associated with estrogen therapy. Caution should also be used in patients with cerebral vascular or coronary artery disease.
- Glucose Tolerance—Because glucose tolerance may be decreased, diabetic patients should be carefully observed while receiving Estramustine .
- Elevated Blood Pressure—Because hypertension may occur, blood pressure should be monitored periodically.
Precautions
General
- Fluid Retention. Exacerbation of preexisting or incipient peripheral edema or congestive heart disease has been seen in some patients receiving therapy with Estramustine Capsules. Other conditions which might be influenced by fluid retention, such as epilepsy, migraine, or renal dysfunction, require careful observation.
- Estramustine may be poorly metabolized in patients with impaired liver function and should be administered with caution in such patients.
- Because Estramustine may influence the metabolism of calcium and phosphorus, it should be used with caution in patients with metabolic bone diseases that are associated with hypercalcemia or in patients with renal insufficiency. Patients with prostate cancer and osteoblastic metastases are at risk for hypocalcemia and should have calcium levels closely monitored.
- Gynecomastia and impotence are known estrogenic effects.
- Allergic reactions and angioedema at times involving the airway have been reported.
Information for the Patient
- Because of the possibility of mutagenic effects, patients should be advised to use contraceptive measures.
Laboratory Tests
- Certain endocrine and liver function tests may be affected by estrogen-containing drugs. Estramustine may depress testosterone levels. Abnormalities of hepatic enzymes and of bilirubin have occurred in patients receiving Estramustine . Such tests should be done at appropriate intervals during therapy and repeated after the drug has been withdrawn for two months.
Food/Drug Interaction
- Milk, milk products, and calcium-rich foods or drugs may impair the absorption of Estramustine .
Adverse Reactions
Clinical Trials Experience
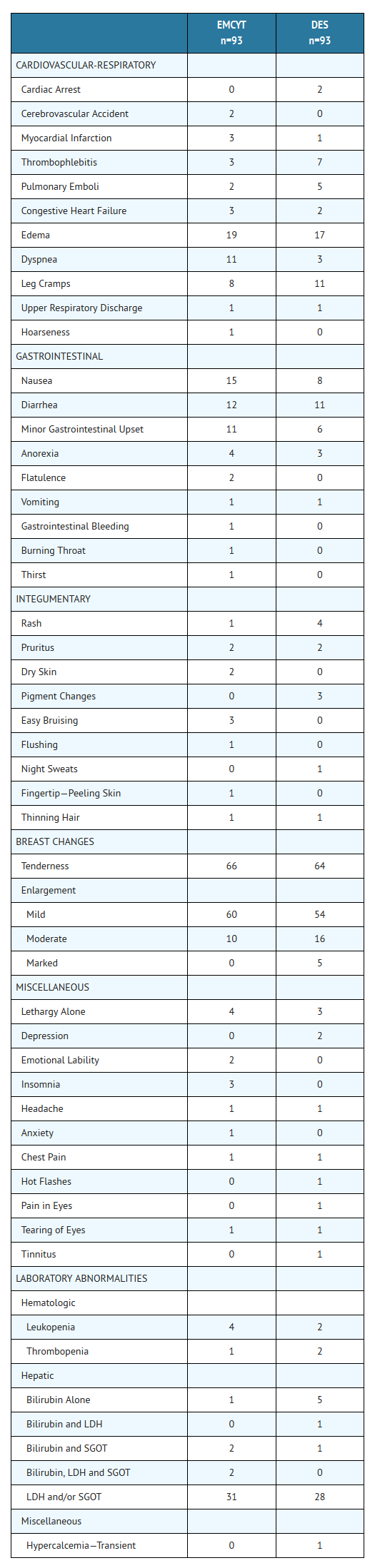
- In a randomized, double-blind trial comparing therapy with Estramustine Capsules in 93 patients (11.5 to 15.9 mg/kg/day) or diethylstilbestrol (DES) in 93 patients (3.0 mg/day), the following adverse effects were reported:
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Estramustine in the drug label.
Drug Interactions
There is limited information regarding Estramustine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Estramustine in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Estramustine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Estramustine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Estramustine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Estramustine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Estramustine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Estramustine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Estramustine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Estramustine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Estramustine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Estramustine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Estramustine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Estramustine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Estramustine in the drug label.
Overdosage
- Although there has been no experience with overdosage to date, it is reasonable to expect that such episodes may produce pronounced manifestations of the known adverse reactions. In the event of overdosage, the gastric contents should be evacuated by gastric lavage and symptomatic therapy should be initiated. Hematologic and hepatic parameters should be monitored for at least 6 weeks after overdosage of Estramustine Capsules
Pharmacology
| |
| |
Estramustine
| |
| Systematic (IUPAC) name | |
| (17β)-17-Hydroxyestra-1(10),2,4-trien-3-yl bis(2-chloroethyl)carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 440.403 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 75%[1] |
| Metabolism | Hepatic to estradiol and estrone[1] |
| Half life | 15-24 hours[1] |
| Excretion | Faeces (2.9-4.8%)[1] |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Prolonged treatment with estramustine phosphate produces elevated total plasma concentrations of estradiol that fall within ranges similar to the elevated estradiol levels found in prostatic cancer patients given conventional estradiol therapy.
Structure
- Estramustine phosphate sodium, an antineoplastic agent, is an off-white powder readily soluble in water. Estramustine Capsules are white and opaque, each containing estramustine phosphate sodium as the disodium salt monohydrate equivalent to 140 mg estramustine phosphate, for oral administration. Each capsule also contains magnesium stearate, silicon dioxide, sodium lauryl sulfate, and talc. Gelatin capsule shells contain the following pigment: titanium dioxide.
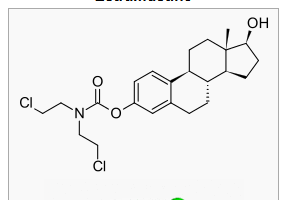
- Chemically, estramustine phosphate sodium is estra-1,3,5(10)-triene-3,17-diol(17β)-,3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. It is also referred to as estradiol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. Estramustine phosphate sodium has an empiric formula of C23H30Cl2NNa2O6P•H2O, a calculated molecular weight of 582.4, and the following structural formula:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Estramustine in the drug label.
Pharmacokinetics
- Estramustine phosphate (Figure 1) is a molecule combining estradiol and nornitrogen mustard by a carbamate link. The molecule is phosphorylated to make it water soluble.There is limited information regarding Pharmacokinetics of Estramustine in the drug label.
- Estramustine phosphate taken orally is readily dephosphorylated during absorption, and the major metabolites in plasma are estramustine (Figure 2), the estrone analog (Figure 3), estradiol, and estrone.
- Estrogenic effects, as demonstrated by changes in circulating levels of steroids and pituitary hormones, are similar in patients treated with either estramustine phosphate or conventional estradiol.
- The metabolic urinary patterns of the estradiol moiety of estramustine phosphate and estradiol itself are very similar, although the metabolites derived from estramustine phosphate are excreted at a slower rate.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term continuous administration of estrogens in certain animal species increases the frequency of carcinomas of the breast and liver. Compounds structurally similar to Estramustine are carcinogenic in mice. Carcinogenic studies of Estramustine have not been conducted in man. Although testing by the Ames method failed to demonstrate mutagenicity for estramustine phosphate sodium, it is known that both estradiol and nitrogen mustard are mutagenic. For this reason and because some patients who had been impotent while on estrogen therapy have regained potency while taking Estramustine , the patient should be advised to use contraceptive measures.
Clinical Studies
There is limited information regarding Clinical Studies of Estramustine in the drug label.
How Supplied
- White opaque capsules, each containing estramustine phosphate sodium as the disodium salt monohydrate equivalent to 140 mg estramustine phosphate—bottle of 100 (NDC 0013-0132-02).
Storage
NOTE
- Estramustine Capsules should be stored in the refrigerator at 36° to 46°F (2° to 8°C).
Images
Drug Images
{{#ask: Page Name::Estramustine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Estramustine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Estramustine in the drug label.
Precautions with Alcohol
- Alcohol-Estramustine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Emcyt®
Look-Alike Drug Names
There is limited information regarding Estramustine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Estramustine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Estramustine |Label Name=Estramustine03.png
}}
{{#subobject:
|Label Page=Estramustine |Label Name=Estramustine04.png
}}
{{#subobject:
|Label Page=Estramustine |Label Name=Estramustine05.png
}}
