Emtricitabine and tenofovir disoproxil fumarate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 212: | Line 212: | ||
(Description regarding monitoring, from ''Warnings'' section) | (Description regarding monitoring, from ''Warnings'' section) | ||
| | |overdose=If overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary. | ||
'''Emtricitabine:''' Limited clinical experience is available at doses higher than the therapeutic dose of [[emtricitabine]]. In one clinical pharmacology trial, single doses of [[emtricitabine]] 1200 mg were administered to 11 subjects. No severe adverse reactions were reported. | |||
Hemodialysis treatment removes approximately 30% of the [[emtricitabine]] dose over a 3-hour dialysis period starting within 1.5 hours of [[emtricitabine]] dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). It is not known whether [[emtricitabine]] can be removed by peritoneal dialysis. | |||
'''Tenofovir Disoproxil Fumarate:''' Limited clinical experience at doses higher than the therapeutic dose of [[tenofovir]] disoproxil fumarate 300 mg is available. In one trial, 600 mg [[tenofovir]] disoproxil fumarate was administered to 8 subjects orally for 28 days, and no severe adverse reactions were reported. The effects of higher doses are not known. | |||
Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of [[tenofovir]] disoproxil fumarate, a four-hour [[hemodialysis]] session removed approximately 10% of the administered [[tenofovir]] dose. | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = | | verifiedrevid = | ||
Revision as of 13:47, 13 August 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS, POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE FOR PRE-EXPOSURE PROPHYLAXIS (PrEP) IN UNDIAGNOSED EARLY HIV-1 INFECTION
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS, POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE FOR PRE-EXPOSURE PROPHYLAXIS (PrEP) IN UNDIAGNOSED EARLY HIV-1 INFECTION: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of emtricitabine and tenofovir disoproxil fumarate, in combination with other antiretrovirals.
Emtricitabine and tenofovir disoproxil fumarate is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of emtricitabine and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued emtricitabine and tenofovir disoproxil fumarate. Therefore, hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are infected with HBV and discontinue emtricitabine and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted. Emtricitabine and tenofovir disoproxil fumarate used for a PrEP indication must only be prescribed to individuals confirmed to be HIV-negative immediately prior to initiating and periodically (at least every 3 months) during use. Drug-resistant HIV-1 variants have been identified with use of emtricitabine and tenofovir disoproxil fumarate for a PrEP indication following undetected acute HIV-1 infection. Do not initiate emtricitabine and tenofovir disoproxil fumarate for a PrEP indication if signs or symptoms of acute HIV-1 infection are present unless negative infection status is confirmed. |
Overview
Emtricitabine and tenofovir disoproxil fumarate is a human immunodeficiency virus 1 non-nucleoside analog reverse transcriptase inhibitor that is FDA approved for the treatment of HIV-1 Infection, pre-exposure prophylaxis to HIV-1. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, lactic acidosis, abdominal pain, diarrhea, nausea, serum amylase raised, backache, myalgia, osteopenia, dizziness, headache, insomnia, peripheral neuropathy, depression, dream disorder, pneumonia, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose for Treatment of HIV-1 Infection
The recommended dose of emtricitabine and tenofovir disoproxil fumarate in adults and in pediatric patients 12 years of age and older with body weight greater than or equal to 35 kg (greater than or equal to 77 lb) is one tablet (containing 200 mg of emtricitabine and 300 mg of tenofovir disoproxil fumarate) once daily taken orally with or without food.
Recommended Dose for Pre-exposure Prophylaxis
The dose of emtricitabine and tenofovir disoproxil fumarate in HIV-1 uninfected adults is one tablet (containing 200 mg of emtricitabine and 300 mg of tenofovir disoproxil fumarate) once daily taken orally with or without food.
Dose Adjustment for Renal Impairment
Treatment of HIV-1 Infection
Significantly increased drug exposures occurred when emtricitabine or tenofovir disoproxil fumarate were administered to subjects with moderate to severe renal impairment. Therefore, adjust the dosing interval of emtricitabine and tenofovir disoproxil fumarate in HIV-1 infected adult patients with baseline creatinine clearance 30–49 mL/min using the recommendations in Table 1. These dosing interval recommendations are based on modeling of single-dose pharmacokinetic data in non-HIV infected subjects. The safety and effectiveness of these dosing interval adjustment recommendations have not been clinically evaluated in patients with moderate renal impairment, therefore clinical response to treatment and renal function should be closely monitored in these patients.
No dose adjustment is necessary for HIV-1 infected patients with mild renal impairment (creatinine clearance 50–80 mL/min). No data are available to make dose recommendations in pediatric patients with renal impairment.
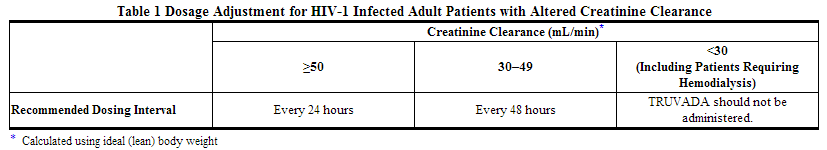
Routine monitoring of estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein should be performed in all individuals with mild renal impairment.
Pre-exposure Prophylaxis
Do not use emtricitabine and tenofovir disoproxil fumarate for a PrEP indication in HIV-1 uninfected individuals with estimated creatinine clearance below 60 mL/min.
Routine monitoring of estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein should be performed in all individuals with mild renal impairment. If a decrease in estimated creatinine clearance is observed in uninfected individuals while using emtricitabine and tenofovir disoproxil fumarate for PrEP, evaluate potential causes and re-assess potential risks and benefits of continued use.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Emtricitabine and tenofovir disoproxil fumarate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Emtricitabine and tenofovir disoproxil fumarate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Emtricitabine and tenofovir disoproxil fumarate FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Emtricitabine and tenofovir disoproxil fumarate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Emtricitabine and tenofovir disoproxil fumarate in pediatric patients.
Contraindications
Do not use emtricitabine and tenofovir disoproxil fumarate for pre-exposure prophylaxis in individuals with unknown or positive HIV-1 status. Emtricitabine and tenofovir disoproxil fumarate should be used in HIV-infected patients only in combination with other antiretroviral agents.
Warnings
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS, POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE FOR PRE-EXPOSURE PROPHYLAXIS (PrEP) IN UNDIAGNOSED EARLY HIV-1 INFECTION
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS, POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE FOR PRE-EXPOSURE PROPHYLAXIS (PrEP) IN UNDIAGNOSED EARLY HIV-1 INFECTION: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of emtricitabine and tenofovir disoproxil fumarate, in combination with other antiretrovirals.
Emtricitabine and tenofovir disoproxil fumarate is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of emtricitabine and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued emtricitabine and tenofovir disoproxil fumarate. Therefore, hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are infected with HBV and discontinue emtricitabine and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted. Emtricitabine and tenofovir disoproxil fumarate used for a PrEP indication must only be prescribed to individuals confirmed to be HIV-negative immediately prior to initiating and periodically (at least every 3 months) during use. Drug-resistant HIV-1 variants have been identified with use of emtricitabine and tenofovir disoproxil fumarate for a PrEP indication following undetected acute HIV-1 infection. Do not initiate emtricitabine and tenofovir disoproxil fumarate for a PrEP indication if signs or symptoms of acute HIV-1 infection are present unless negative infection status is confirmed. |
Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of emtricitabine and tenofovir disoproxil fumarate, in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient or uninfected individual with known risk factors for liver disease; however, cases have also been reported in HIV-1 infected patients with no known risk factors. Treatment with emtricitabine and tenofovir disoproxil fumarate should be suspended in any patient or uninfected individual who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
HBV Infection
It is recommended that all individuals be tested for the presence of chronic hepatitis B virus (HBV) before initiating emtricitabine and tenofovir disoproxil fumarate. emtricitabine and tenofovir disoproxil fumarate is not approved for the treatment of chronic HBV infection and the safety and efficacy of emtricitabine and tenofovir disoproxil fumarate have not been established in patients infected with HBV. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued emtricitabine and tenofovir disoproxil fumarate. In some patients infected with HBV and treated with EMTRIVA, the exacerbations of hepatitis B were associated with liver decompensation and liver failure. Patients who are infected with HBV should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with emtricitabine and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted. HBV -uninfected individuals should be offered vaccination.
New Onset or Worsening Renal Impairment
Emtricitabine and tenofovir are principally eliminated by the kidney. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of VIREAD.
It is recommended that estimated creatinine clearance be assessed in all individuals prior to initiating therapy and as clinically appropriate during therapy with emtricitabine and tenofovir disoproxil fumarate. In patients at risk of renal dysfunction, including patients who have previously experienced renal events while receiving HEPSERA®, it is recommended that estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein be assessed prior to initiation of emtricitabine and tenofovir disoproxil fumarate, and periodically during emtricitabine and tenofovir disoproxil fumarate therapy.
Emtricitabine and tenofovir disoproxil fumarate should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs)) [See Drug Interactions (7.3)]. Cases of acute renal failure after initiation of high dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on tenofovir DF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
Treatment of HIV-1 Infection
Dosing interval adjustment of emtricitabine and tenofovir disoproxil fumarate and close monitoring of renal function are recommended in all patients with estimated creatinine clearance 30–49 mL/min, [See Dosage and Administration (2.3)]. No safety or efficacy data are available in patients with renal impairment who received emtricitabine and tenofovir disoproxil fumarate using these dosing guidelines, so the potential benefit of emtricitabine and tenofovir disoproxil fumarate therapy should be assessed against the potential risk of renal toxicity. Emtricitabine and tenofovir disoproxil fumarate should not be administered to patients with estimated creatinine clearance below 30 mL/min or patients requiring hemodialysis.
Pre-exposure Prophylaxis
Emtricitabine and tenofovir disoproxil fumarate for a PrEP indication should not be used if estimated creatinine clearance is less than 60 mL/min. If a decrease in estimated creatinine clearance is observed in uninfected individuals while using emtricitabine and tenofovir disoproxil fumarate for PrEP, evaluate potential causes and re-assess potential risks and benefits of continued use.
Coadministration with Other Products
Emtricitabine and tenofovir disoproxil fumarate is a fixed-dose combination of emtricitabine and tenofovir disoproxil fumarate. Do not coadminister emtricitabine and tenofovir disoproxil fumarate with ATRIPLA, COMPLERA, EMTRIVA, STRIBILD, or VIREAD. Due to similarities between emtricitabine and lamivudine, do not coadminister emtricitabine and tenofovir disoproxil fumarate with other drugs containing lamivudine, including Combivir (lamivudine/zidovudine), Epivir or Epivir-HBV (lamivudine), Epzicom (abacavir sulfate/lamivudine), or Trizivir (abacavir sulfate/lamivudine/zidovudine). Do not coadminister emtricitabine and tenofovir disoproxil fumarate with HEPSERA (adefovir dipivoxil).
Bone Effects of Tenofovir DF
Bone Mineral Density
In clinical trials in HIV-1 infected adults and in a clinical trial of HIV-1 uninfected individuals, tenofovir DF was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving tenofovir DF.
Clinical trials evaluating tenofovir DF in pediatric and adolescent subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1 infected subjects aged 2 years to less than 18 years, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the tenofovir DF treated HIV-1 infected pediatric subjects as compared to the control groups. Similar trends were observed in chronic hepatitis B infected adolescent subjects aged 12 years to less than 18 years. In all pediatric trials, skeletal growth (height) appeared to be unaffected. For more information, please consult the VIREAD prescribing information.
The effects of tenofovir DF-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown. Assessment of BMD should be considered for adult and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial. If bone abnormalities are suspected then appropriate consultation should be obtained.
Mineralization Defects
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with the use of tenofovir DF. Arthralgias and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving products containing tenofovir DF.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in HIV-1 infected patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in HIV-1 infected patients treated with combination antiretroviral therapy, including emtricitabine and tenofovir disoproxil fumarate. During the initial phase of combination antiretroviral treatment, HIV-1 infected patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Early Virologic Failure
Clinical trials in HIV-1 infected subjects have demonstrated that certain regimens that only contain three nucleoside reverse transcriptase inhibitors (NRTI) are generally less effective than triple drug regimens containing two NRTIs in combination with either a non-nucleoside reverse transcriptase inhibitor or a HIV-1 protease inhibitor. In particular, early virological failure and high rates of resistance substitutions have been reported. Triple nucleoside regimens should therefore be used with caution. Patients on a therapy utilizing a triple nucleoside-only regimen should be carefully monitored and considered for treatment modification.
Comprehensive Management to Reduce the Risk of Acquiring HIV-1
Use emtricitabine and tenofovir disoproxil fumarate for pre-exposure prophylaxis only as part of a comprehensive prevention strategy that includes other prevention measures, such as safer sex practices, because emtricitabine and tenofovir disoproxil fumarate is not always effective in preventing the acquisition of HIV-1.
Counsel uninfected individuals about safer sex practices that include consistent and correct use of condoms, knowledge of their HIV-1 status and that of their partner(s), and regular testing for other sexually transmitted infections that can facilitate HIV-1 transmission (such as syphilis and gonorrhea). Inform uninfected individuals about and support their efforts in reducing sexual risk behavior.
Use emtricitabine and tenofovir disoproxil fumarate to reduce the risk of acquiring HIV-1 only in individuals confirmed to be HIV-negative. HIV-1 resistance substitutions may emerge in individuals with undetected HIV-1 infection who are taking only emtricitabine and tenofovir disoproxil fumarate, because emtricitabine and tenofovir disoproxil fumarate alone does not constitute a complete treatment regimen for HIV-1 treatment; therefore, care should be taken to minimize drug exposure in HIV-infected individuals.
Many HIV-1 tests, such as rapid tests, detect anti-HIV antibodies and may not identify HIV-1 during the acute stage of infection. Prior to initiating emtricitabine and tenofovir disoproxil fumarate for a PrEP indication, evaluate seronegative individuals for current or recent signs or symptoms consistent with acute viral infections (e.g., fever, fatigue, myalgia, skin rash, etc.) and ask about potential exposure events (e.g., unprotected, or condom broke during sex with an HIV-1 infected partner) that may have occurred within the last month.
If clinical symptoms consistent with acute viral infection are present and recent (<1 month) exposures are suspected, delay starting PrEP for at least one month and reconfirm HIV-1 status or use a test approved by the FDA as an aid in the diagnosis of HIV-1 infection, including acute or primary HIV-1 infection. While using emtricitabine and tenofovir disoproxil fumarate for a PrEP indication, HIV-1 screening tests should be repeated at least every 3 months. If symptoms consistent with acute HIV-1 infection develop following a potential exposure event, PrEP should be discontinued until negative infection status is confirmed using a test approved by the FDA as an aid in the diagnosis of HIV-1, including acute or primary HIV-1 infection.
Counsel uninfected individuals to strictly adhere to the recommended emtricitabine and tenofovir disoproxil fumarate dosing schedule. The effectiveness of emtricitabine and tenofovir disoproxil fumarate in reducing the risk of acquiring HIV-1 is strongly correlated with adherence as demonstrated by measurable drug levels in clinical trials
Adverse Reactions
Clinical Trials Experience
Adverse Reactions from Clinical Trials Experience in HIV-1 Infected Subjects
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Adult Subjects
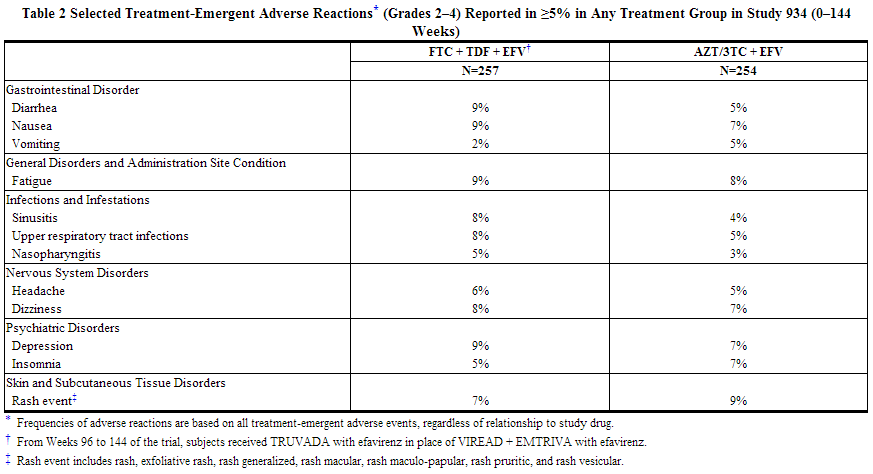
The most common adverse reactions (incidence greater than or equal to 10%, any severity) occurring in Study 934, an active-controlled clinical trial of efavirenz, emtricitabine, and tenofovir disoproxil fumarate, include diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. See also Table 2 for the frequency of treatment-emergent adverse reactions (Grades 2–4) occurring in greater than or equal to 5% of subjects treated in any treatment group in this trial.
Skin discoloration, manifested by hyperpigmentation on the palms and/or soles, was generally mild and asymptomatic. The mechanism and clinical significance are unknown. Study 934 - Treatment Emergent Adverse Reactions: In Study 934, 511 antiretroviral-naive subjects received either tenofovir disoproxil fumarate + emtricitabine administered in combination with efavirenz (N=257) or zidovudine/lamivudine administered in combination with efavirenz (N=254) for 144 weeks. Subjects had a mean age of 40 years (range 20 to 73 years) and were predominantly male (88%). Overall, 65% were White, 17% were Black, and 13% were Hispanic. Adverse reactions observed in this trial were generally consistent with those seen in other trials in treatment-experienced or treatment-naive subjects receiving tenofovir disoproxil fumarate and/or emtricitabine (Table 2).
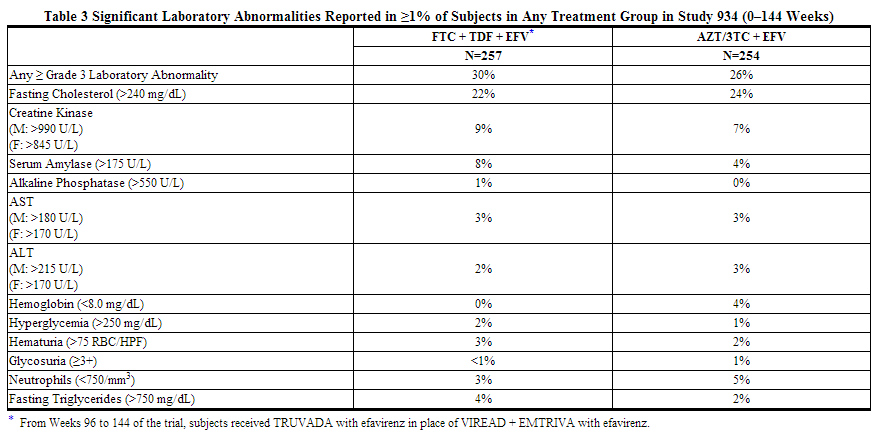
Laboratory Abnormalities: Laboratory abnormalities observed in this trial were generally consistent with those seen in other trials of tenofovir disoproxil fumarate and/or emtricitabine (Table 3).
In addition to the events described above for Study 934, other adverse reactions that occurred in at least 5% of subjects receiving emtricitabine or tenofovir disoproxil fumarate with other antiretroviral agents in clinical trials include anxiety, arthralgia, increased cough, dyspepsia, fever, myalgia, pain, abdominal pain, back pain, paresthesia, peripheral neuropathy (including peripheral neuritis and neuropathy), pneumonia, and rhinitis.
In addition to the laboratory abnormalities described above for Study 934, Grades 3–4 laboratory abnormalities of increased bilirubin (>2.5 × ULN), increased pancreatic amylase (>2.0 × ULN), increased or decreased serum glucose (<40 or >250 mg/dL), and increased serum lipase (>2.0 × ULN) occurred in up to 3% of subjects treated with emtricitabine or tenofovir disoproxil fumarate with other antiretroviral agents in clinical trials.
Clinical Trials in Pediatric Subjects 12 Years of Age and Older
Emtricitabine: In addition to the adverse reactions reported in adults, anemia and hyperpigmentation were observed in 7% and 32%, respectively, of pediatric subjects (3 months to less than 18 years of age) who received treatment with emtricitabine in the larger of two open-label, uncontrolled pediatric trials (N=116). For additional information, please consult the emtricitabine prescribing information.
Tenofovir Disoproxil Fumarate: In a pediatric clinical trial conducted in subjects 12 to less than 18 years of age, the adverse reactions observed in pediatric subjects who received treatment with VIREAD were consistent with those observed in clinical trials of tenofovir disoproxil fumarate in adults.
Adverse Reactions from Clinical Trial Experience in HIV-1 Uninfected Adult Subjects
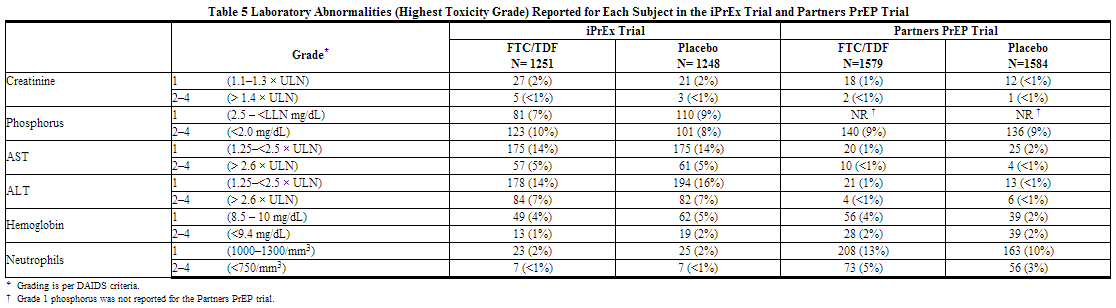
No new adverse reactions to TRUVADA were identified from two randomized placebo-controlled clinical trials (iPrEx, Partners PrEP) in which 2830 HIV-1 uninfected adults received TRUVADA once daily for pre-exposure prophylaxis. Subjects were followed for a median of 71 weeks and 87 weeks, respectively. These trials enrolled HIV-negative individuals ranging in age from 18 to 67 years. The iPrEx trial enrolled only males or transgender females of Hispanic/Latino (72%), White (18%), Black (9%) and Asian (5%) race. The Partners PrEP trial enrolled both males (61–64% across treatment groups) and females in Kenya and Uganda. Table 4 provides a list of all adverse events that occurred ≥2% of subjects in any treatment group in the iPrEx and Partners PrEP trials.
Laboratory Abnormalities: Table 5 provides a list of laboratory abnormalities observed in both trials. Six subjects in the TDF-containing arms of the Partners PrEP trial discontinued participation in the study due to an increase in blood creatinine compared with no discontinuations in the placebo group. One subject in the emtricitabine, and tenofovir disoproxil fumarate arm of the iPrEx trial discontinued from the study due to an increase in blood creatinine and another due to low phosphorous.
In addition to the laboratory abnormalities described above, Grade 1 proteinuria (1+) occurred in 6% of subjects receiving emtricitabine, and tenofovir disoproxil fumarate in the iPrEx trial. Grades 2–3 proteinuria (2–4+) and glycosuria (3+) occurred in less than 1% of subjects treated with emtricitabine, and tenofovir disoproxil fumarate in the iPrEx trial and Partners PrEP trial.

Changes in Bone Mineral Density
In clinical trials of HIV-1 uninfected individuals, decreases in BMD were observed. In the iPrEx trial, a substudy of 503 subjects found mean changes from baseline in BMD ranging from -0.4% to -1.0% across total hip, spine, femoral neck, and trochanter in the emtricitabine, and tenofovir disoproxil fumarate group compared with the placebo group, which returned toward baseline after discontinuation of treatment. Thirteen percent of subjects receiving emtricitabine, and tenofovir disoproxil fumarate vs. 6% of subjects receiving placebo lost at least 5% of BMD at the spine during treatment. Bone fractures were reported in 1.7% of the emtricitabine, and tenofovir disoproxil fumarate group compared with 1.4% in the placebo group. No correlation between BMD and fractures was noted. The Partners PrEP trial found similar fracture rates between treatment and placebo groups (0.8% and 0.6%, respectively). No BMD evaluations were conducted during this trial.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of tenofovir disoproxil fumarate. No additional adverse reactions have been identified during postapproval use of emtricitabine. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune System Disorders: allergic reaction, including angioedema
- Metabolism and Nutrition Disorders: lactic acidosis, hypokalemia, hypophosphatemia
- Respiratory, Thoracic, and Mediastinal Disorders: dyspnea
- Gastrointestinal Disorders: pancreatitis, increased amylase, abdominal pain
- Hepatobiliary Disorders: hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT, GGT)
- Skin and Subcutaneous Tissue Disorders: rash
- Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis, osteomalacia(manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
- Renal and Urinary Disorders: acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
- General Disorders and Administration Site Conditions: asthenia
The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
Drug Interactions
No drug interaction trials have been conducted using emtricitabine and tenofovir disoproxil fumarate tablets. Drug interaction trials have been conducted with emtricitabine and tenofovir disoproxil fumarate, the components of emtricitabine and tenofovir disoproxil fumarate. This section describes clinically relevant drug interactions observed with emtricitabine and tenofovir disoproxil fumarate.
Didanosine
Coadministration of emtricitabine and tenofovir disoproxil fumarate and didanosine should be undertaken with caution and patients receiving this combination should be monitored closely for didanosine-associated adverse reactions. Didanosine should be discontinued in patients who develop didanosine-associated adverse reactions.
When tenofovir disoproxil fumarate was administered with didanosine the Cmax and AUC of didanosine increased significantly. The mechanism of this interaction is unknown. Higher didanosine concentrations could potentiate didanosine-associated adverse reactions, including pancreatitis, and neuropathy. Suppression of CD4+ cell counts has been observed in patients receiving tenofovir DF with didanosine 400 mg daily.
In patients weighing greater than 60 kg, the didanosine dose should be reduced to 250 mg when it is coadministered with emtricitabine and tenofovir disoproxil fumarate. Data are not available to recommend a dose adjustment of didanosine for adult or pediatric patients weighing less than 60 kg. When coadministered, emtricitabine and tenofovir disoproxil fumarate and Videx EC may be taken under fasted conditions or with a light meal (less than 400 kcal, 20% fat).
HIV-1 Protease Inhibitors
Tenofovir decreases the AUC and Cmin of atazanavir. When coadministered with emtricitabine and tenofovir disoproxil fumarate, it is recommended that atazanavir 300 mg is given with ritonavir 100 mg. emtricitabine and tenofovir disoproxil fumarate should not be coadministered with atazanavir without ritonavir.
Lopinavir/ritonavir, atazanavir coadministered with ritonavir, and darunavir coadministered with ritonavir have been shown to increase tenofovir concentrations. Tenofovir disoproxil fumarate is a substrate of P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP) transporters. When tenofovir disoproxil fumarate is co-administered with an inhibitor of these transporters, an increase in absorption may be observed. Patients receiving emtricitabine and tenofovir disoproxil fumarate concomitantly with lopinavir/ritonavir, ritonavir-boosted atazanavir, or ritonavir-boosted darunavir should be monitored for tenofovir-associated adverse reactions. Emtricitabine and tenofovir disoproxil fumarate should be discontinued in patients who develop tenofovir-associated adverse reactions.
Drugs Affecting Renal Function
Emtricitabine and tenofovir are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion. No drug-drug interactions due to competition for renal excretion have been observed; however, coadministration of emtricitabine and tenofovir disoproxil fumarate with drugs that are eliminated by active tubular secretion may increase concentrations of emtricitabine, tenofovir, and/or the coadministered drug. Some examples include, but are not limited to acyclovir, adefovir dipivoxil, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs. Drugs that decrease renal function may increase concentrations of emtricitabine and/or tenofovir.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Antiretroviral Pregnancy Registry: To monitor fetal outcomes of pregnant women exposed to emtricitabine and tenofovir disoproxil fumarate, an Antiretroviral Pregnancy Registry (APR) has been established. Healthcare providers are encouraged to register patients by calling 1-800-258-4263.
Risk Summary
Emtricitabine and tenofovir disoproxil fumarate has been evaluated in a limited number of women during pregnancy and postpartum. Available human and animal data suggest that emtricitabine and tenofovir disoproxil fumarate does not increase the risk of major birth defects overall compared to the background rate. There are, however, no adequate and well-controlled trials in pregnant women. Because the studies in humans cannot rule out the possibility of harm, emtricitabine and tenofovir disoproxil fumarate should be used during pregnancy only if clearly needed. If an uninfected individual becomes pregnant while taking emtricitabine and tenofovir disoproxil fumarate for a PrEP indication, careful consideration should be given to whether use of emtricitabine and tenofovir disoproxil fumarate should be continued, taking into account the potential increased risk of HIV-1 infection during pregnancy.
Clinical Considerations=
As of July 2011, the APR has received prospective reports of 764 and 1219 exposures to emtricitabine and tenofovir containing regimens, respectively in the first trimester, 321 and 455 exposures, respectively, in second trimester, and 140 and 257 exposures, respectively, in the third trimester. Birth defects occurred in 18 of 764 (2.4%) live births for emtricitabine-containing regimens and 27 of 1219 (2.2%) live births for tenofovir-containing regimens (first trimester exposure) and 10 of 461 (2.2%) live births for emtricitabine-containing regimens and 15 of 714 (2.1%) live births for tenofovir-containing regimens (second/third trimester exposure). Among pregnant women in the U.S. reference population, the background rate of birth defects is 2.7%. There was no association between emtricitabine or tenofovir and overall birth defects observed in the APR.
Animal Data
Emtricitabine
The incidence of fetal variations and malformations was not increased in embryofetal toxicity studies performed with emtricitabine in mice at exposures (AUC) approximately 60-fold higher and in rabbits at approximately 120-fold higher than human exposures at the recommended daily dose.
Tenofovir Disoproxil Fumarate
Reproduction studies have been performed in rats and rabbits at doses up to 14 and 19 times the human dose based on body surface area comparisons and revealed no evidence of impaired fertility or harm to the fetus due to tenofovir.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Emtricitabine and tenofovir disoproxil fumarate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Emtricitabine and tenofovir disoproxil fumarate during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV-1.
Studies in humans have shown that both tenofovir and emtricitabine are excreted in human milk. Because the risks of low level exposure to emtricitabine and tenofovir to infants are unknown, mothers should be instructed not to breast-feed if they are receiving emtricitabine and tenofovir disoproxil fumarate, whether they are taking emtricitabine and tenofovir disoproxil fumarate for treatment or to reduce the risk of acquiring HIV-1.
Emtricitabine
Samples of breast milk obtained from five HIV-1 infected mothers show that emtricitabine is secreted in human milk. Breastfeeding infants whose mothers are being treated with emtricitabine may be at risk for developing viral resistance to emtricitabine. Other emtricitabine-associated risks in infants breastfed by mothers being treated with emtricitabine are unknown.
Tenofovir Disoproxil Fumarate
Samples of breast milk obtained from five HIV-1 infected mothers show that tenofoviris secreted in human milk. Tenofovir-associated risks, including the risk of viral resistance to tenofovir, in infants breastfed by mothers being treated with tenofovir disoproxil fumarate are unknown.
Pediatric Use
Emtricitabine and tenofovir disoproxil fumarateshould only be administered to HIV-1 infected pediatric patients 12 years of age and older with body weight greater than or equal to 35 kg. Because it is a fixed-dose combination tablet, emtricitabine and tenofovir disoproxil fumarate cannot be adjusted for patients of lower age and weight. Safety and efficacy have not been established in pediatric patients less than 12 years of age or weighing less than 35 kg.
Geriatic Use
Clinical trials of emtricitabine or tenofovir disoproxil fumarate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for the elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Emtricitabine and tenofovir disoproxil fumarate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Emtricitabine and tenofovir disoproxil fumarate with respect to specific racial populations.
Renal Impairment
Treatment of HIV-1 Infection
The dosing interval for emtricitabine or tenofovir disoproxil fumarate should be modified in HIV-infected adult patients with estimated creatinine clearance of 30–49 mL/min. Emtricitabine or tenofovir disoproxil fumarate should not be used in patients with estimated creatinine clearance below 30 mL/min and in patients with end-stage renal disease requiring dialysis.
Pre-exposure Prophylaxis
Emtricitabine or tenofovir disoproxil fumarate for a PrEP indication should not be used in HIV-1 uninfected individuals with estimated creatinine clearance below 60 mL/min. If a decrease in estimated creatinine clearance is observed in uninfected individuals while using emtricitabine or tenofovir disoproxil fumarate for PrEP, evaluate potential causes and re-assess potential risks and benefits of continued use.
Hepatic Impairment
There is no FDA guidance on the use of Emtricitabine and tenofovir disoproxil fumarate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Emtricitabine and tenofovir disoproxil fumarate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Emtricitabine and tenofovir disoproxil fumarate in patients who are immunocompromised.
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Emtricitabine and tenofovir disoproxil fumarate and IV administrations.
Overdosage
If overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Emtricitabine: Limited clinical experience is available at doses higher than the therapeutic dose of emtricitabine. In one clinical pharmacology trial, single doses of emtricitabine 1200 mg were administered to 11 subjects. No severe adverse reactions were reported.
Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3-hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). It is not known whether emtricitabine can be removed by peritoneal dialysis.
Tenofovir Disoproxil Fumarate: Limited clinical experience at doses higher than the therapeutic dose of tenofovir disoproxil fumarate 300 mg is available. In one trial, 600 mg tenofovir disoproxil fumarate was administered to 8 subjects orally for 28 days, and no severe adverse reactions were reported. The effects of higher doses are not known.
Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of tenofovir disoproxil fumarate, a four-hour hemodialysis session removed approximately 10% of the administered tenofovir dose.
Pharmacology
Emtricitabine and tenofovir disoproxil fumarate
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Emtricitabine and tenofovir disoproxil fumarate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Emtricitabine and tenofovir disoproxil fumarate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Emtricitabine and tenofovir disoproxil fumarate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Emtricitabine and tenofovir disoproxil fumarate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Emtricitabine and tenofovir disoproxil fumarate Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.